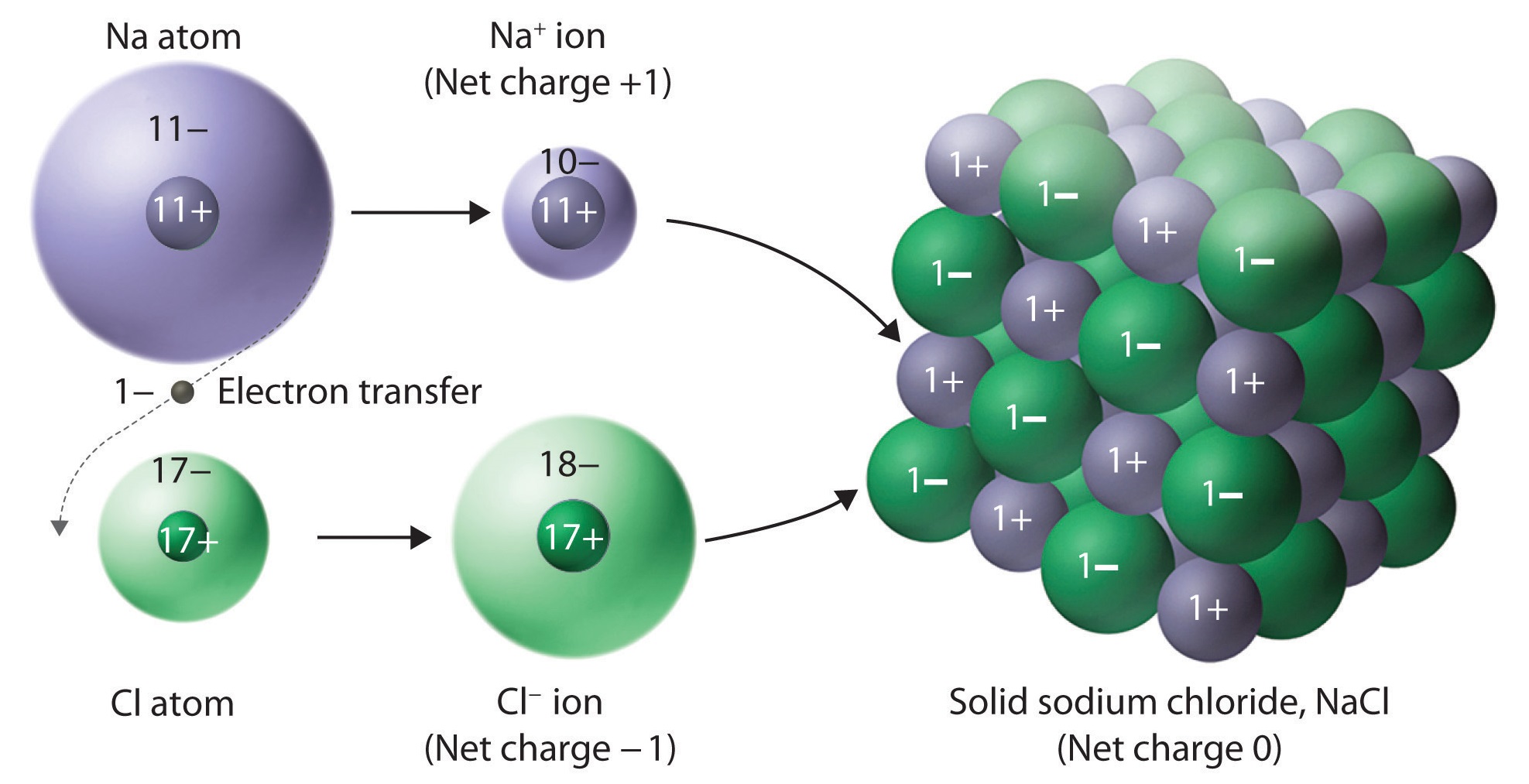
A Positive Ion Is Formed When
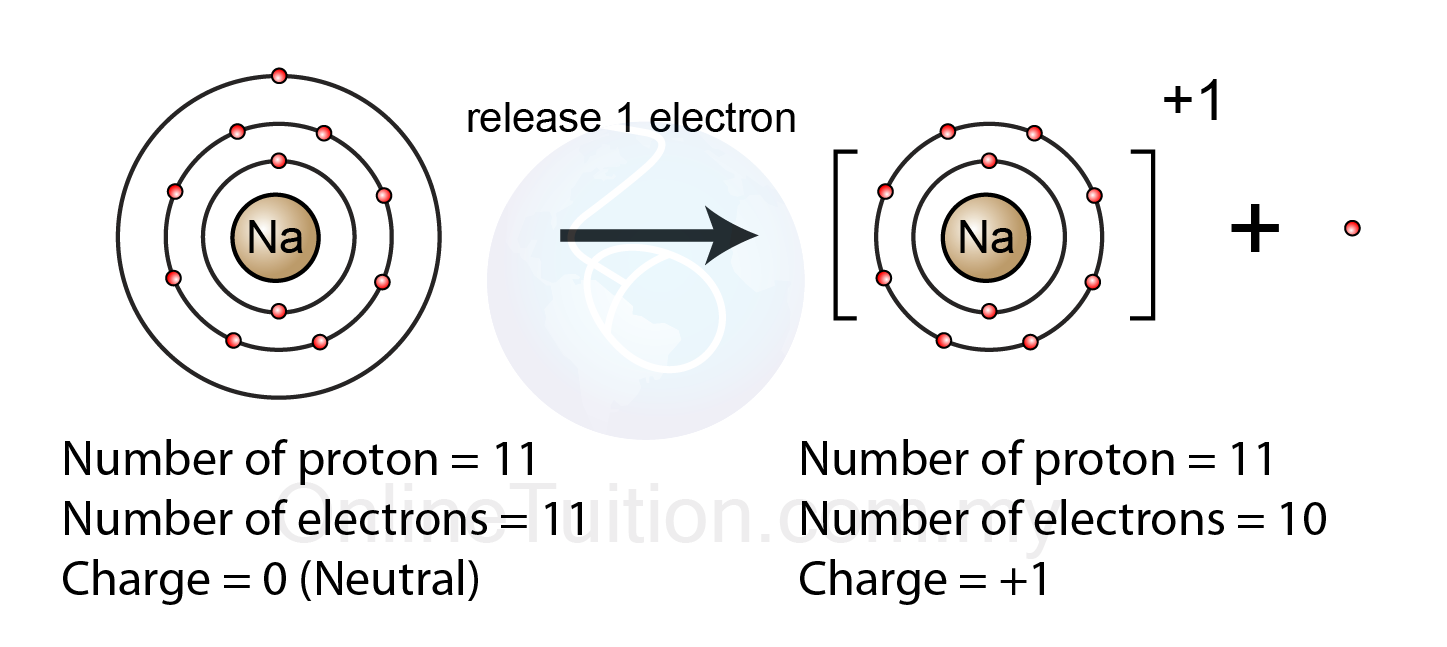
A Positive Ion Is Formed When - Positive and negative ions are formed by gaining or losing electrons from neutral atoms. A negative ion loses an electron. An ion always has a charge that is positive (+) or negative (−). An atom that has gained or lost one or more electrons is called an ion. A positive ion is formed when a. Ionic formulas balance the total positive and. A neutral atom gains an electron. Ionic compounds have positive ions and negative ions. A neutral atom loses an electron. Ions form when atoms lose or gain electrons.
Positive and negative ions are formed by gaining or losing electrons from neutral atoms. An ion always has a charge that is positive (+) or negative (−). Ions form when atoms lose or gain electrons. Ionic formulas balance the total positive and. Ionic compounds have positive ions and negative ions. A neutral atom loses an electron. A neutral atom gains an electron. An atom that has gained or lost one or more electrons is called an ion. A negative ion loses an electron. A positive ion is formed when a.
Ions form when atoms lose or gain electrons. An atom that has gained or lost one or more electrons is called an ion. Ionic formulas balance the total positive and. A neutral atom loses an electron. An ion always has a charge that is positive (+) or negative (−). Positive and negative ions are formed by gaining or losing electrons from neutral atoms. A negative ion loses an electron. A positive ion is formed when a. A neutral atom gains an electron. Ionic compounds have positive ions and negative ions.
Periodic Table Which Groups Of Elements Tend To Form Positive Ions
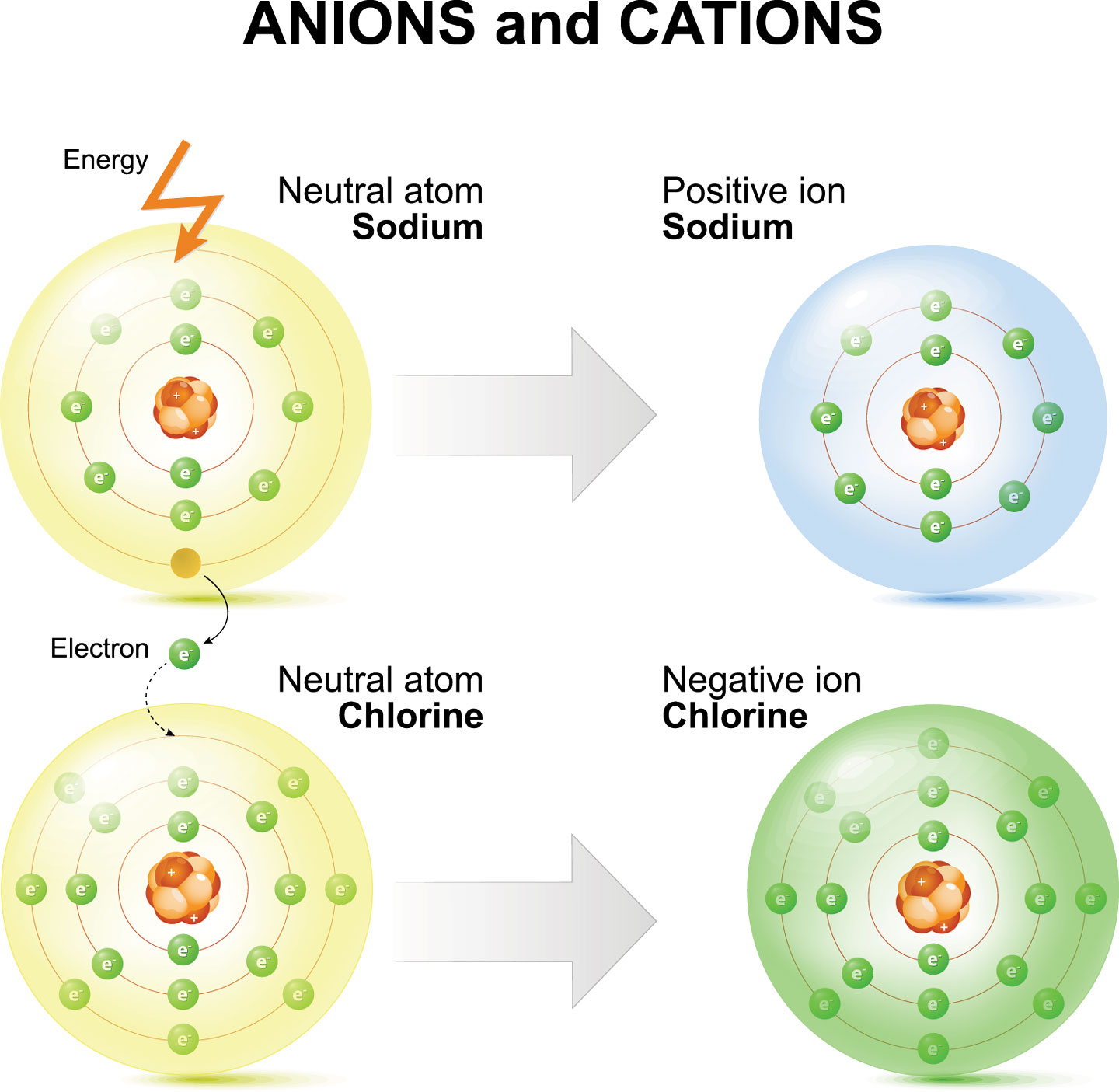
An ion always has a charge that is positive (+) or negative (−). Ions form when atoms lose or gain electrons. A neutral atom loses an electron. Ionic compounds have positive ions and negative ions. Positive and negative ions are formed by gaining or losing electrons from neutral atoms.
5.2.1 Formation of Ion Revision.my
A neutral atom gains an electron. A negative ion loses an electron. An atom that has gained or lost one or more electrons is called an ion. Positive and negative ions are formed by gaining or losing electrons from neutral atoms. An ion always has a charge that is positive (+) or negative (−).
Bonding and Structure* — the science sauce
A neutral atom gains an electron. Ions form when atoms lose or gain electrons. Positive and negative ions are formed by gaining or losing electrons from neutral atoms. A positive ion is formed when a. A negative ion loses an electron.
Atomic Structure / Periodic Table ppt download
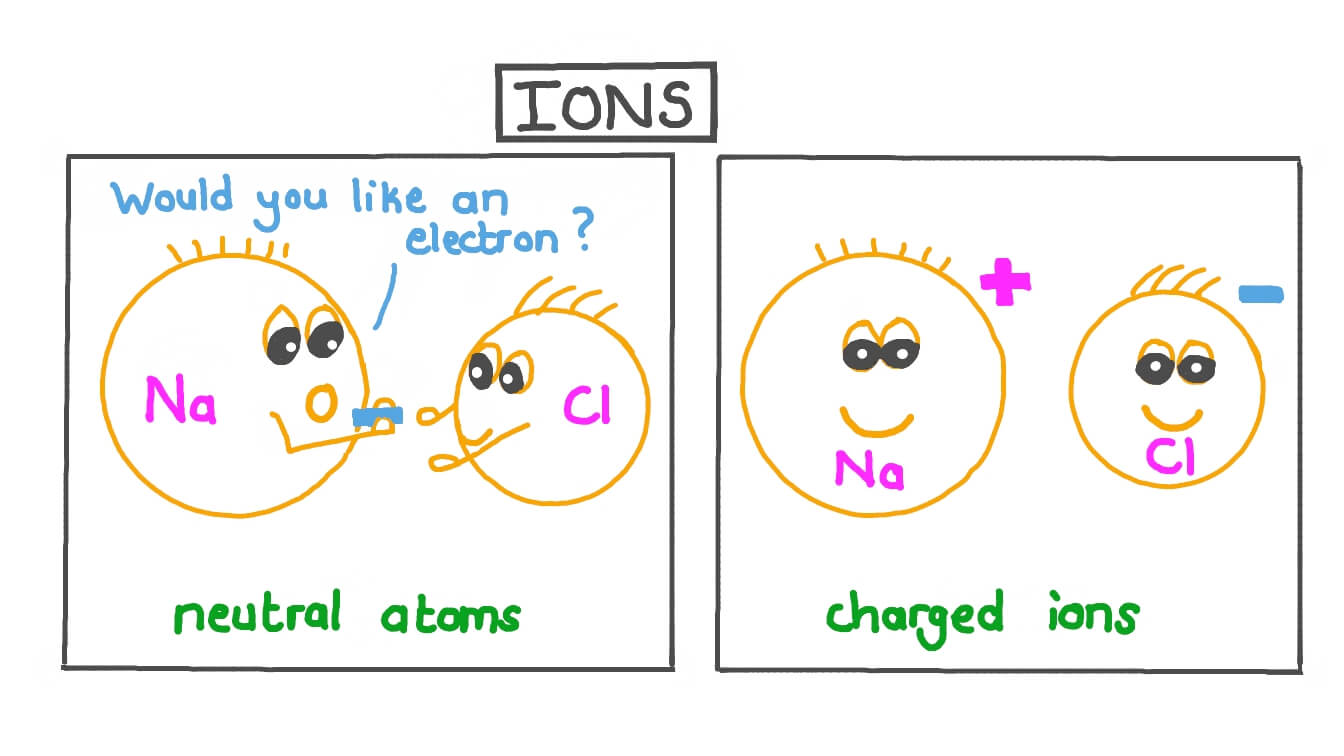
A negative ion loses an electron. An ion always has a charge that is positive (+) or negative (−). A neutral atom gains an electron. A neutral atom loses an electron. Ionic compounds have positive ions and negative ions.
5.2.1 Formation of Ion Revision.my
A neutral atom gains an electron. A negative ion loses an electron. An ion always has a charge that is positive (+) or negative (−). Ions form when atoms lose or gain electrons. Ionic formulas balance the total positive and.
design a positive ion with a charge of 2 annalealyon
A neutral atom gains an electron. An atom that has gained or lost one or more electrons is called an ion. Positive and negative ions are formed by gaining or losing electrons from neutral atoms. A negative ion loses an electron. A neutral atom loses an electron.
Draw the structure of the stable positive ion formed when an acid
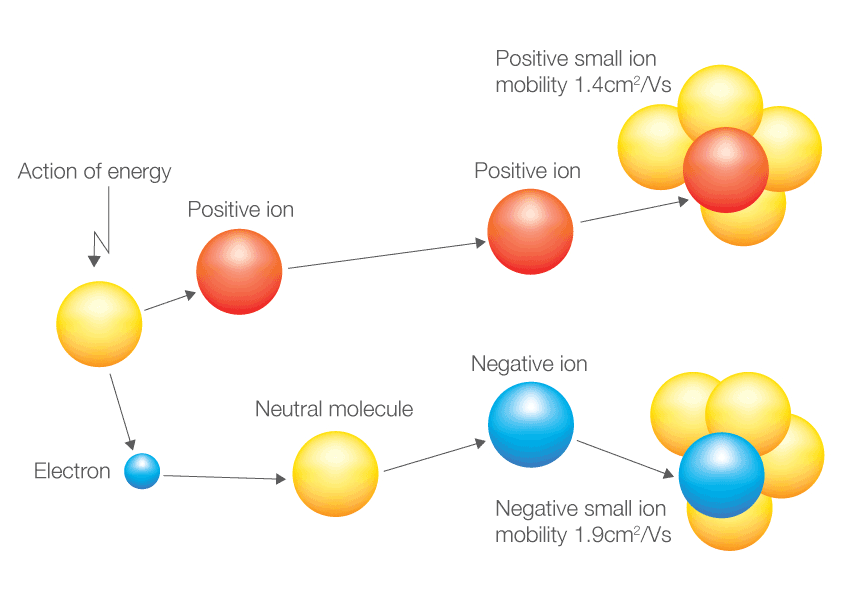
Positive and negative ions are formed by gaining or losing electrons from neutral atoms. An atom that has gained or lost one or more electrons is called an ion. A positive ion is formed when a. Ionic formulas balance the total positive and. An ion always has a charge that is positive (+) or negative (−).
Lesson Video Ions Nagwa
Ions form when atoms lose or gain electrons. An ion always has a charge that is positive (+) or negative (−). A neutral atom gains an electron. A negative ion loses an electron. An atom that has gained or lost one or more electrons is called an ion.
All about positive and negative ions?
An ion always has a charge that is positive (+) or negative (−). Positive and negative ions are formed by gaining or losing electrons from neutral atoms. A negative ion loses an electron. An atom that has gained or lost one or more electrons is called an ion. Ionic compounds have positive ions and negative ions.
20.12 Engineering Chemistry LibreTexts
Ionic formulas balance the total positive and. An atom that has gained or lost one or more electrons is called an ion. A neutral atom gains an electron. Ions form when atoms lose or gain electrons. An ion always has a charge that is positive (+) or negative (−).
Ionic Compounds Have Positive Ions And Negative Ions.
Positive and negative ions are formed by gaining or losing electrons from neutral atoms. Ions form when atoms lose or gain electrons. A neutral atom gains an electron. A neutral atom loses an electron.
A Negative Ion Loses An Electron.
An ion always has a charge that is positive (+) or negative (−). An atom that has gained or lost one or more electrons is called an ion. A positive ion is formed when a. Ionic formulas balance the total positive and.