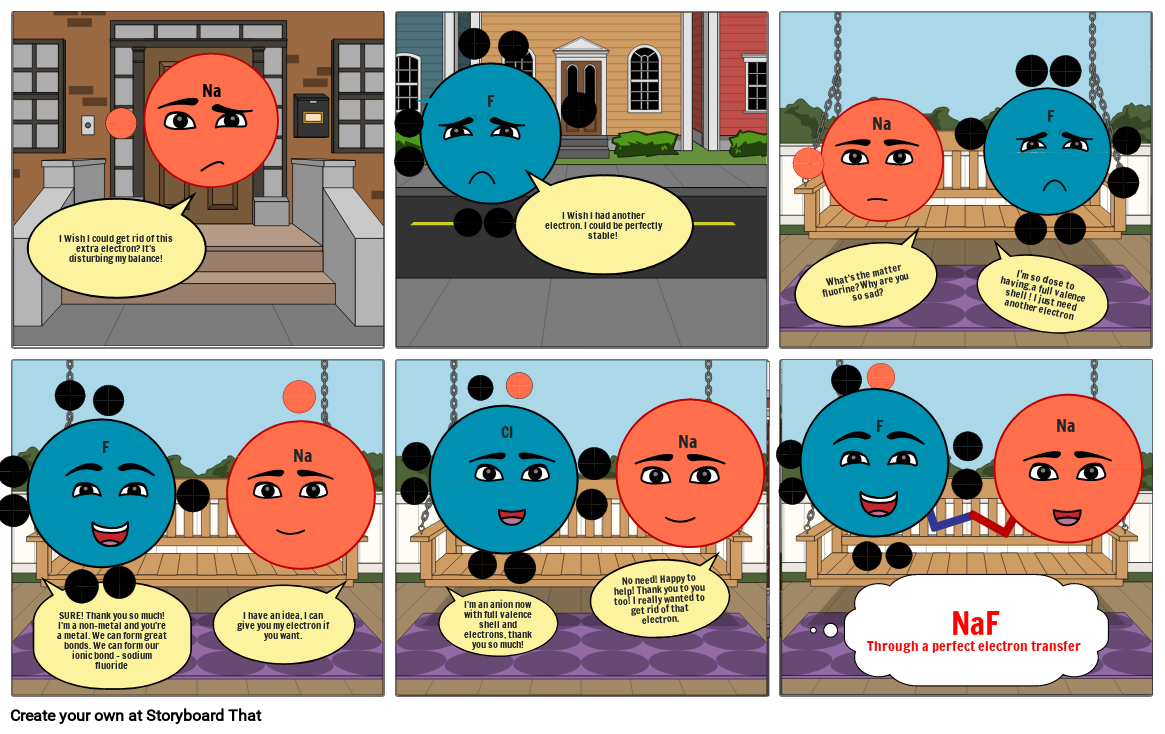
An Ionic Bond Forms Between A
An Ionic Bond Forms Between A - An ionic bond forms between a ____ ion with a positive charge and a ____ ion with a negative charge Such a bond forms when the valence (outermost) electrons of. Ionic bonds form between atoms with large differences in electronegativity, whereas covalent bonds formed between atoms with smaller differences in electronegativity. Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Check all of the boxes that apply. An ionic bond, also known as an electrovalent bond, is a type of chemical bond formed due to the electrostatic attraction between oppositely charged ions in a compound or molecule. Ionic bonds form between metal atoms and other metal atoms. Ionic bonds form between metal atoms and nonmetal atoms. How and why do ionic bonds form?
An ionic bond, also known as an electrovalent bond, is a type of chemical bond formed due to the electrostatic attraction between oppositely charged ions in a compound or molecule. How and why do ionic bonds form? Ionic bonds form between atoms with large differences in electronegativity, whereas covalent bonds formed between atoms with smaller differences in electronegativity. An ionic bond forms between a ____ ion with a positive charge and a ____ ion with a negative charge Such a bond forms when the valence (outermost) electrons of. Ionic bonds form between metal atoms and nonmetal atoms. Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Ionic bonds form between metal atoms and other metal atoms. Check all of the boxes that apply.
Such a bond forms when the valence (outermost) electrons of. Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Ionic bonds form between metal atoms and other metal atoms. Check all of the boxes that apply. Ionic bonds form between metal atoms and nonmetal atoms. An ionic bond forms between a ____ ion with a positive charge and a ____ ion with a negative charge How and why do ionic bonds form? An ionic bond, also known as an electrovalent bond, is a type of chemical bond formed due to the electrostatic attraction between oppositely charged ions in a compound or molecule. Ionic bonds form between atoms with large differences in electronegativity, whereas covalent bonds formed between atoms with smaller differences in electronegativity.
Ionic Bonding Storyboard by cceeeb96
An ionic bond, also known as an electrovalent bond, is a type of chemical bond formed due to the electrostatic attraction between oppositely charged ions in a compound or molecule. Check all of the boxes that apply. How and why do ionic bonds form? An ionic bond forms between a ____ ion with a positive charge and a ____ ion.
ionic bond Definition, Properties, Examples, & Facts Britannica
An ionic bond, also known as an electrovalent bond, is a type of chemical bond formed due to the electrostatic attraction between oppositely charged ions in a compound or molecule. Ionic bonds form between metal atoms and nonmetal atoms. An ionic bond forms between a ____ ion with a positive charge and a ____ ion with a negative charge Check.
10+ Ionic Bonding Diagram Robhosking Diagram
How and why do ionic bonds form? Ionic bonds form between metal atoms and nonmetal atoms. Ionic bonds form between atoms with large differences in electronegativity, whereas covalent bonds formed between atoms with smaller differences in electronegativity. An ionic bond, also known as an electrovalent bond, is a type of chemical bond formed due to the electrostatic attraction between oppositely.
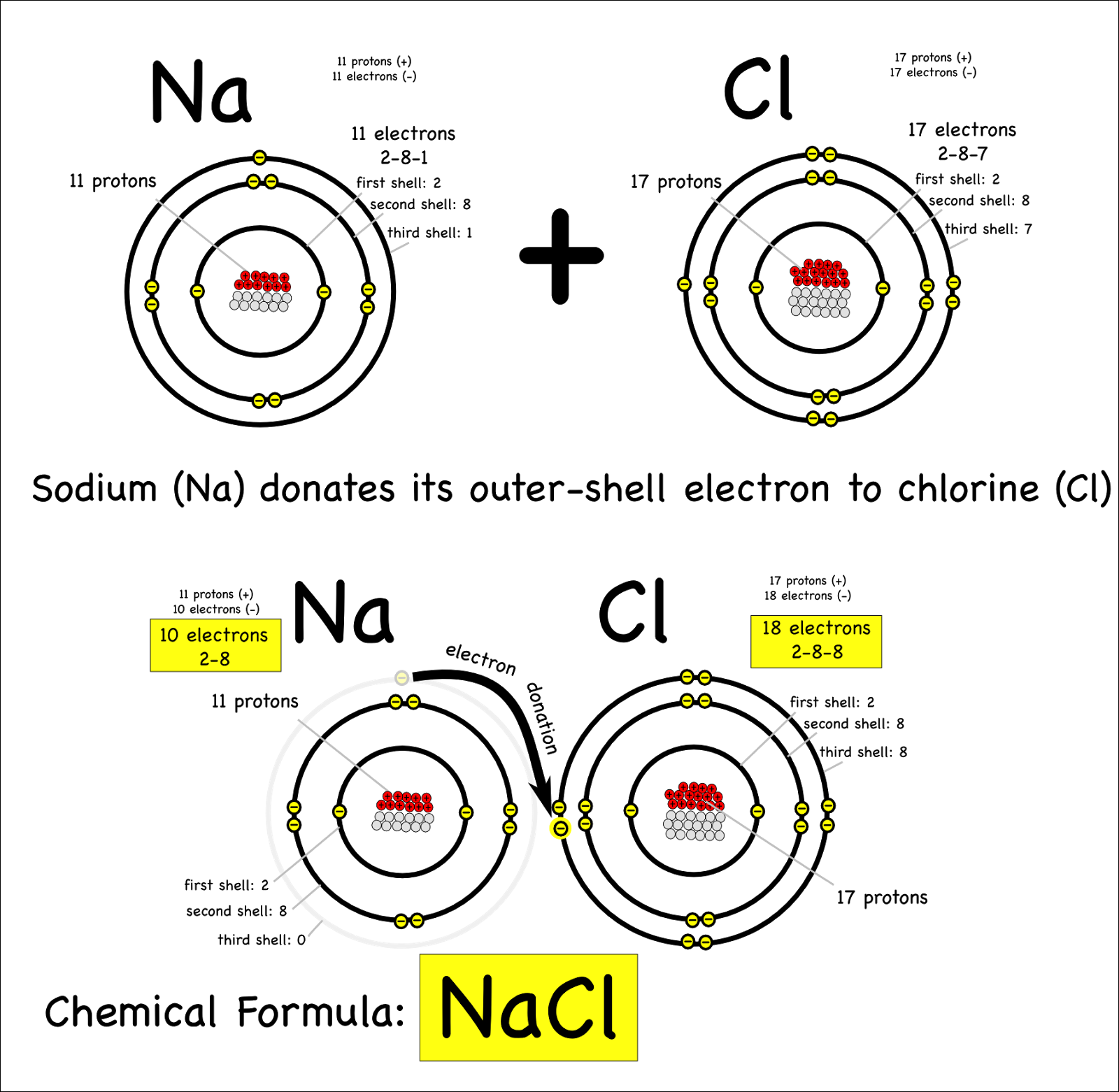
(PPT) Chapter 6 Review. An ionic bond forms between a ___________ and a
An ionic bond, also known as an electrovalent bond, is a type of chemical bond formed due to the electrostatic attraction between oppositely charged ions in a compound or molecule. Such a bond forms when the valence (outermost) electrons of. How and why do ionic bonds form? Check all of the boxes that apply. Ionic bond, type of linkage formed.
Fourth Grade GC August 2013
How and why do ionic bonds form? Ionic bonds form between metal atoms and nonmetal atoms. Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Ionic bonds form between atoms with large differences in electronegativity, whereas covalent bonds formed between atoms with smaller differences in electronegativity. Check all of the boxes.
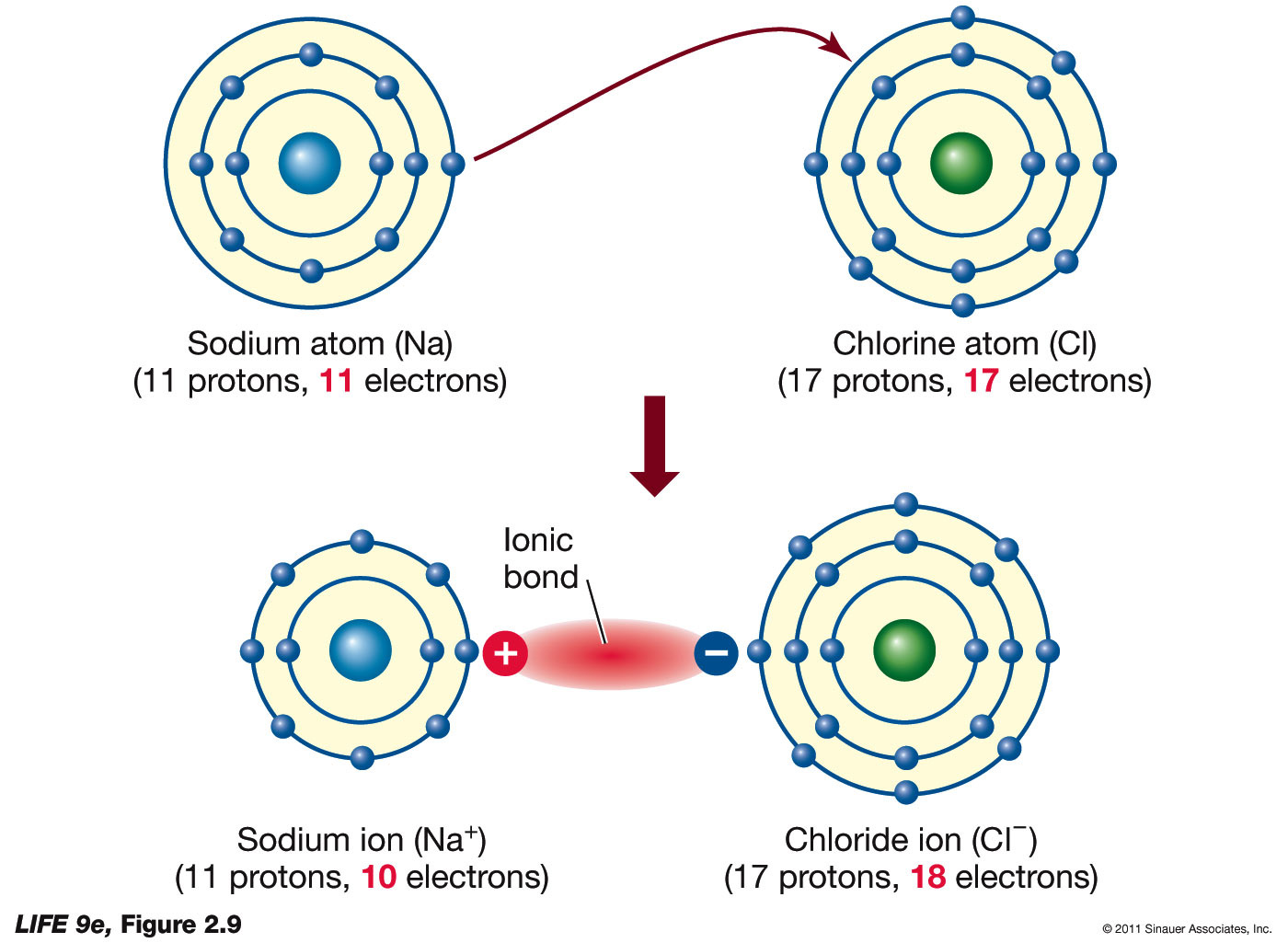
SOLVED An ionic bond forms between what two types of atoms?
Such a bond forms when the valence (outermost) electrons of. Ionic bonds form between metal atoms and other metal atoms. How and why do ionic bonds form? Ionic bonds form between metal atoms and nonmetal atoms. Ionic bonds form between atoms with large differences in electronegativity, whereas covalent bonds formed between atoms with smaller differences in electronegativity.
Ionic Bond Definition and Examples
Ionic bonds form between metal atoms and other metal atoms. An ionic bond, also known as an electrovalent bond, is a type of chemical bond formed due to the electrostatic attraction between oppositely charged ions in a compound or molecule. Ionic bonds form between metal atoms and nonmetal atoms. Such a bond forms when the valence (outermost) electrons of. How.
(PPTX) Ionic Nomenclature. An ionic bond forms between metals and
Ionic bonds form between metal atoms and other metal atoms. Ionic bonds form between metal atoms and nonmetal atoms. How and why do ionic bonds form? An ionic bond, also known as an electrovalent bond, is a type of chemical bond formed due to the electrostatic attraction between oppositely charged ions in a compound or molecule. An ionic bond forms.
What Is An Ionic Bond Sciencing Ionic Bonding Ionic Chemical Bond
An ionic bond, also known as an electrovalent bond, is a type of chemical bond formed due to the electrostatic attraction between oppositely charged ions in a compound or molecule. Such a bond forms when the valence (outermost) electrons of. An ionic bond forms between a ____ ion with a positive charge and a ____ ion with a negative charge.
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures
An ionic bond, also known as an electrovalent bond, is a type of chemical bond formed due to the electrostatic attraction between oppositely charged ions in a compound or molecule. Ionic bonds form between metal atoms and other metal atoms. Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Check all.
Check All Of The Boxes That Apply.
An ionic bond forms between a ____ ion with a positive charge and a ____ ion with a negative charge How and why do ionic bonds form? An ionic bond, also known as an electrovalent bond, is a type of chemical bond formed due to the electrostatic attraction between oppositely charged ions in a compound or molecule. Ionic bonds form between metal atoms and other metal atoms.
Ionic Bonds Form Between Metal Atoms And Nonmetal Atoms.
Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Such a bond forms when the valence (outermost) electrons of. Ionic bonds form between atoms with large differences in electronegativity, whereas covalent bonds formed between atoms with smaller differences in electronegativity.





.PNG)
