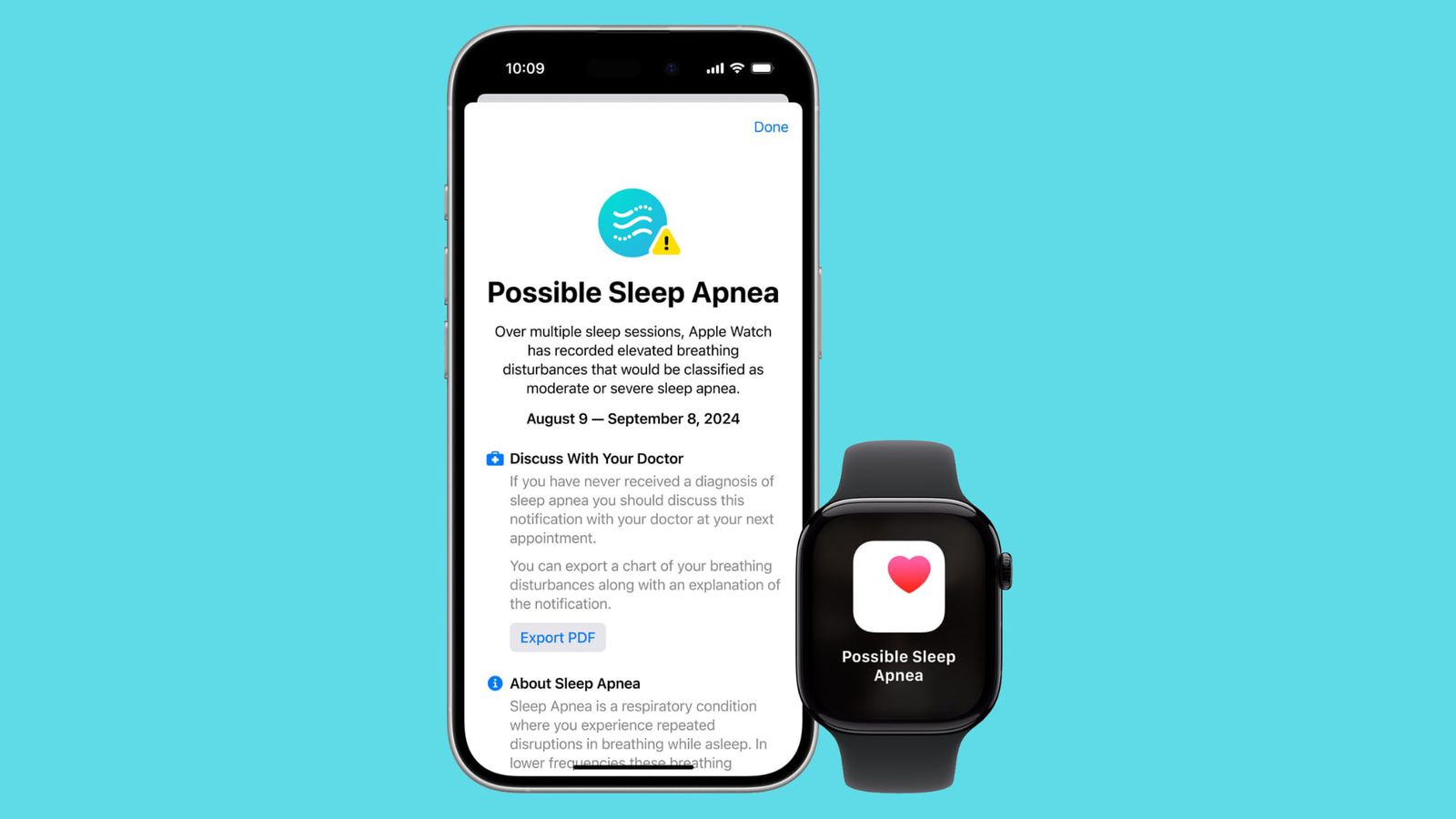
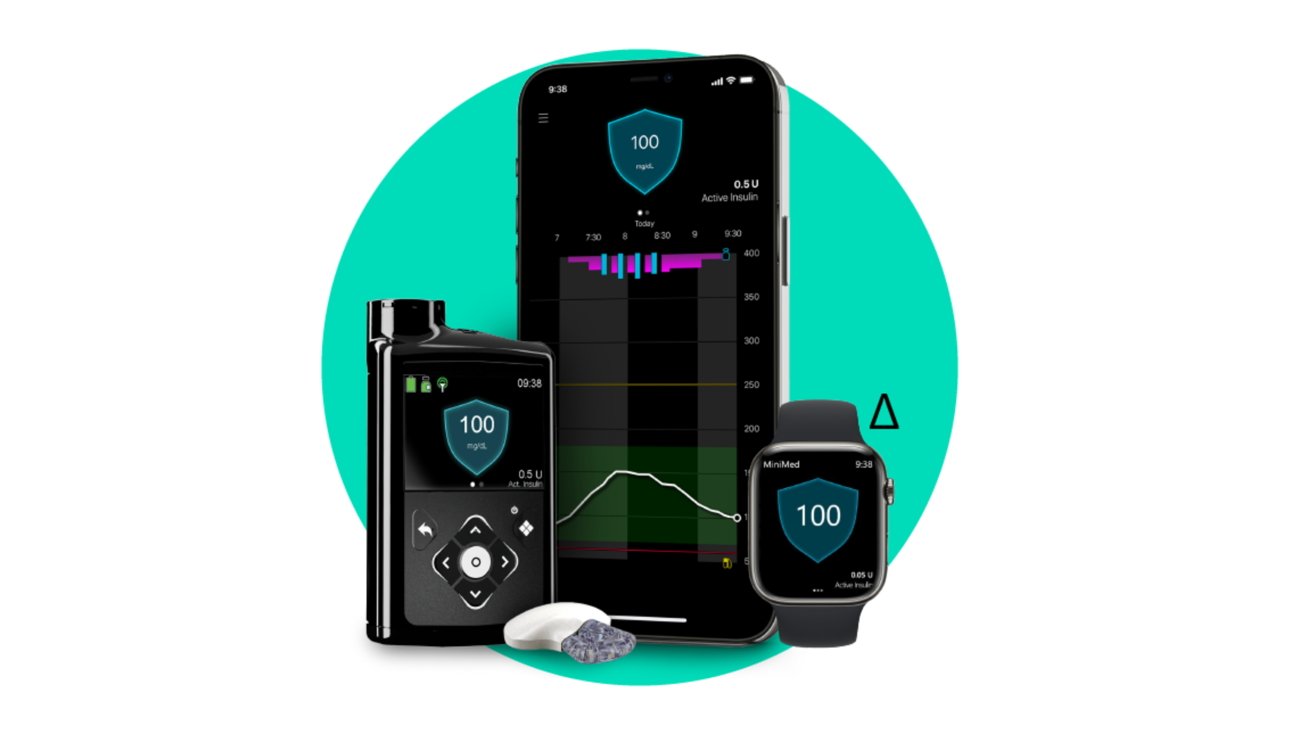
Apple Watch Fda Approval
Apple Watch Fda Approval - Food and drug administration monday published approval for sleep apnea detection on the apple watch. Apple received 510 (k) premarket clearance from the food and drug administration (fda) for a new feature for the os9 version of. Fda officials will accept data collected by apple watch as a secondary endpoint to help assess afib burden in studies of cardiac. The fda has approved the apple watch's atrial fibrillation history feature under its stringent medical device development tools.
The fda has approved the apple watch's atrial fibrillation history feature under its stringent medical device development tools. Fda officials will accept data collected by apple watch as a secondary endpoint to help assess afib burden in studies of cardiac. Apple received 510 (k) premarket clearance from the food and drug administration (fda) for a new feature for the os9 version of. Food and drug administration monday published approval for sleep apnea detection on the apple watch.
Apple received 510 (k) premarket clearance from the food and drug administration (fda) for a new feature for the os9 version of. Food and drug administration monday published approval for sleep apnea detection on the apple watch. Fda officials will accept data collected by apple watch as a secondary endpoint to help assess afib burden in studies of cardiac. The fda has approved the apple watch's atrial fibrillation history feature under its stringent medical device development tools.
Apple Watch can monitor heart rhythms with FDA approval
Fda officials will accept data collected by apple watch as a secondary endpoint to help assess afib burden in studies of cardiac. Food and drug administration monday published approval for sleep apnea detection on the apple watch. The fda has approved the apple watch's atrial fibrillation history feature under its stringent medical device development tools. Apple received 510 (k) premarket.
Apple Watch 心律检测功能获得 FDA 批准 TechBriefly CN
The fda has approved the apple watch's atrial fibrillation history feature under its stringent medical device development tools. Fda officials will accept data collected by apple watch as a secondary endpoint to help assess afib burden in studies of cardiac. Food and drug administration monday published approval for sleep apnea detection on the apple watch. Apple received 510 (k) premarket.
The Apple Watch Series 6’s Blood Oxygen Monitor Didn’t Require FDA
Fda officials will accept data collected by apple watch as a secondary endpoint to help assess afib burden in studies of cardiac. Food and drug administration monday published approval for sleep apnea detection on the apple watch. Apple received 510 (k) premarket clearance from the food and drug administration (fda) for a new feature for the os9 version of. The.
Apple Watch Pro to feature exclusive Bands and Watch Faces
Apple received 510 (k) premarket clearance from the food and drug administration (fda) for a new feature for the os9 version of. Food and drug administration monday published approval for sleep apnea detection on the apple watch. Fda officials will accept data collected by apple watch as a secondary endpoint to help assess afib burden in studies of cardiac. The.
Apple Watch Receives FDA Approval for Sleep Apnea Detection Informed
Apple received 510 (k) premarket clearance from the food and drug administration (fda) for a new feature for the os9 version of. Food and drug administration monday published approval for sleep apnea detection on the apple watch. Fda officials will accept data collected by apple watch as a secondary endpoint to help assess afib burden in studies of cardiac. The.
Apple Watch blood sugar sensor will probably need FDA approval
Apple received 510 (k) premarket clearance from the food and drug administration (fda) for a new feature for the os9 version of. The fda has approved the apple watch's atrial fibrillation history feature under its stringent medical device development tools. Food and drug administration monday published approval for sleep apnea detection on the apple watch. Fda officials will accept data.
WWDC 2022 An Apple Watch Feature Reportedly Got FDA Approval Just
Food and drug administration monday published approval for sleep apnea detection on the apple watch. Fda officials will accept data collected by apple watch as a secondary endpoint to help assess afib burden in studies of cardiac. Apple received 510 (k) premarket clearance from the food and drug administration (fda) for a new feature for the os9 version of. The.
July FDA Approvals to Watch
Fda officials will accept data collected by apple watch as a secondary endpoint to help assess afib burden in studies of cardiac. The fda has approved the apple watch's atrial fibrillation history feature under its stringent medical device development tools. Food and drug administration monday published approval for sleep apnea detection on the apple watch. Apple received 510 (k) premarket.
Medtronic gets FDA approval for new iPhone & Apple Watch connected
Food and drug administration monday published approval for sleep apnea detection on the apple watch. Fda officials will accept data collected by apple watch as a secondary endpoint to help assess afib burden in studies of cardiac. Apple received 510 (k) premarket clearance from the food and drug administration (fda) for a new feature for the os9 version of. The.
Health Canada officially approves Sleep apnea detection on Apple Watch
The fda has approved the apple watch's atrial fibrillation history feature under its stringent medical device development tools. Apple received 510 (k) premarket clearance from the food and drug administration (fda) for a new feature for the os9 version of. Food and drug administration monday published approval for sleep apnea detection on the apple watch. Fda officials will accept data.
Apple Received 510 (K) Premarket Clearance From The Food And Drug Administration (Fda) For A New Feature For The Os9 Version Of.
Food and drug administration monday published approval for sleep apnea detection on the apple watch. The fda has approved the apple watch's atrial fibrillation history feature under its stringent medical device development tools. Fda officials will accept data collected by apple watch as a secondary endpoint to help assess afib burden in studies of cardiac.