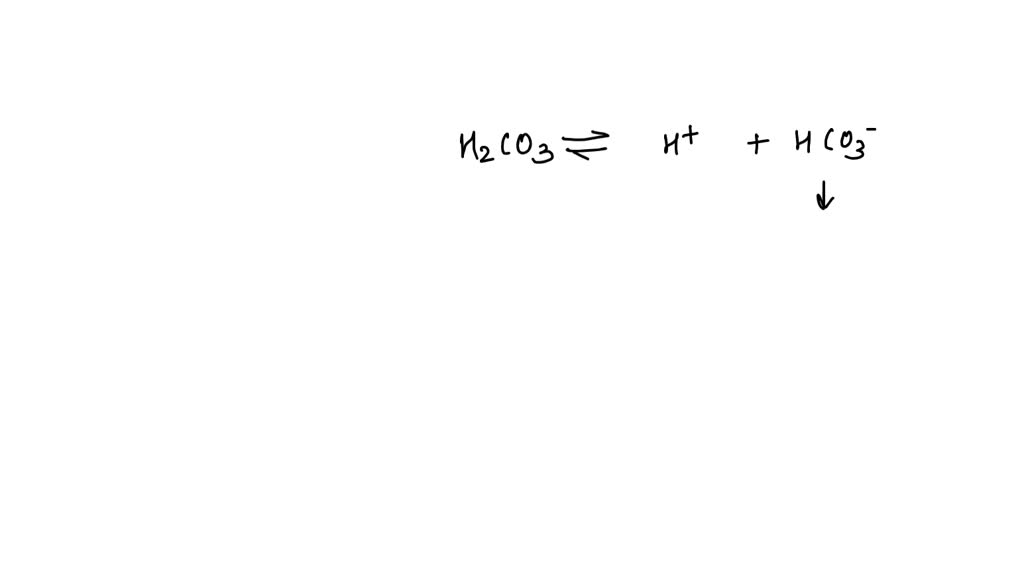Body Fluid Ph Will Rise Dramatically When
Body Fluid Ph Will Rise Dramatically When - An increase in blood co2 levels is followed by a (n) ____ in h+ ions and a (n) ____ in blood ph. A variety of buffering systems exist in the body that helps maintain the ph of the blood and other fluids within a narrow range—between ph 7.35. One question asks what causes. Body fluid ph will rise. Large amounts of bicarbonate are ingested c. [1] the proper balance between the. The diagram illustrates respiratory control of blood. Na+ is excreted by the kidney b. Body fluid ph will rise dramatically when a. Proper physiological functioning depends on a very tight balance between the concentrations of acids and bases in the blood.
[1] the proper balance between the. Body fluid ph will rise. Body fluid ph will rise dramatically when a. Na+ is excreted by the kidney b. Proper physiological functioning depends on a very tight balance between the concentrations of acids and bases in the blood. Large amounts of bicarbonate are ingested c. One question asks what causes. An increase in blood co2 levels is followed by a (n) ____ in h+ ions and a (n) ____ in blood ph. A variety of buffering systems exist in the body that helps maintain the ph of the blood and other fluids within a narrow range—between ph 7.35. The diagram illustrates respiratory control of blood.
The diagram illustrates respiratory control of blood. Large amounts of bicarbonate are ingested c. Na+ is excreted by the kidney b. [1] the proper balance between the. A variety of buffering systems exist in the body that helps maintain the ph of the blood and other fluids within a narrow range—between ph 7.35. An increase in blood co2 levels is followed by a (n) ____ in h+ ions and a (n) ____ in blood ph. Body fluid ph will rise. Body fluid ph will rise dramatically when a. One question asks what causes. Proper physiological functioning depends on a very tight balance between the concentrations of acids and bases in the blood.
pH values of human body fluids. Download Scientific Diagram
Proper physiological functioning depends on a very tight balance between the concentrations of acids and bases in the blood. A variety of buffering systems exist in the body that helps maintain the ph of the blood and other fluids within a narrow range—between ph 7.35. [1] the proper balance between the. Large amounts of bicarbonate are ingested c. The diagram.
SOLVED Body fluid pH will rise dramatically when Select one a. large
Na+ is excreted by the kidney b. An increase in blood co2 levels is followed by a (n) ____ in h+ ions and a (n) ____ in blood ph. Proper physiological functioning depends on a very tight balance between the concentrations of acids and bases in the blood. A variety of buffering systems exist in the body that helps maintain.
Body Fluid pH Balance in Metabolic Health and Possible Benefits of
[1] the proper balance between the. Proper physiological functioning depends on a very tight balance between the concentrations of acids and bases in the blood. Large amounts of bicarbonate are ingested c. An increase in blood co2 levels is followed by a (n) ____ in h+ ions and a (n) ____ in blood ph. One question asks what causes.
Solved Body fluid pH will rise dramatically whenSelect
[1] the proper balance between the. Proper physiological functioning depends on a very tight balance between the concentrations of acids and bases in the blood. An increase in blood co2 levels is followed by a (n) ____ in h+ ions and a (n) ____ in blood ph. The diagram illustrates respiratory control of blood. One question asks what causes.
Body Fluid pH Balance in Metabolic Health and Possible Benefits of
An increase in blood co2 levels is followed by a (n) ____ in h+ ions and a (n) ____ in blood ph. The diagram illustrates respiratory control of blood. Proper physiological functioning depends on a very tight balance between the concentrations of acids and bases in the blood. Body fluid ph will rise. A variety of buffering systems exist in.
important pH values of human body fluids various body fluids pH value
Large amounts of bicarbonate are ingested c. Body fluid ph will rise dramatically when a. An increase in blood co2 levels is followed by a (n) ____ in h+ ions and a (n) ____ in blood ph. Na+ is excreted by the kidney b. Proper physiological functioning depends on a very tight balance between the concentrations of acids and bases.
SOME IMPORTANT pH VALUES OF BODY FLUIDS AND BODY PARTS YouTube
An increase in blood co2 levels is followed by a (n) ____ in h+ ions and a (n) ____ in blood ph. Na+ is excreted by the kidney b. Large amounts of bicarbonate are ingested c. [1] the proper balance between the. Body fluid ph will rise.
pH Homeostasis Concept Anatomy and Physiology JoVe
Body fluid ph will rise dramatically when a. Proper physiological functioning depends on a very tight balance between the concentrations of acids and bases in the blood. [1] the proper balance between the. An increase in blood co2 levels is followed by a (n) ____ in h+ ions and a (n) ____ in blood ph. The diagram illustrates respiratory control.
pH of body fluids pH level in human body Mohit Ranglani Pharmacy
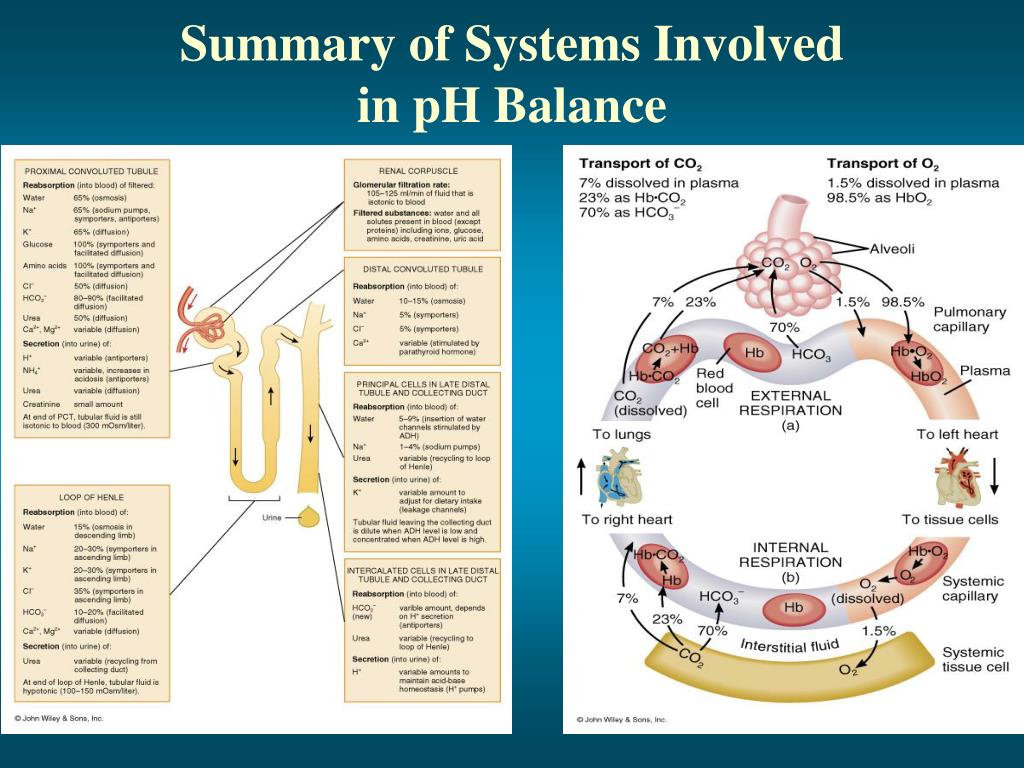
The diagram illustrates respiratory control of blood. [1] the proper balance between the. Proper physiological functioning depends on a very tight balance between the concentrations of acids and bases in the blood. Body fluid ph will rise. Na+ is excreted by the kidney b.
PPT Volume of Body Fluid in the different body compartments
Large amounts of bicarbonate are ingested c. Na+ is excreted by the kidney b. Body fluid ph will rise dramatically when a. Proper physiological functioning depends on a very tight balance between the concentrations of acids and bases in the blood. An increase in blood co2 levels is followed by a (n) ____ in h+ ions and a (n) ____.
One Question Asks What Causes.
Proper physiological functioning depends on a very tight balance between the concentrations of acids and bases in the blood. Body fluid ph will rise. Body fluid ph will rise dramatically when a. Na+ is excreted by the kidney b.
Large Amounts Of Bicarbonate Are Ingested C.
A variety of buffering systems exist in the body that helps maintain the ph of the blood and other fluids within a narrow range—between ph 7.35. An increase in blood co2 levels is followed by a (n) ____ in h+ ions and a (n) ____ in blood ph. [1] the proper balance between the. The diagram illustrates respiratory control of blood.