Can Nh3 Form Hydrogen Bonds
Can Nh3 Form Hydrogen Bonds - However, forming hydrogen bonds is an. The nitrogen atom in ammonia has a lone pair of electrons, which can form a hydrogen bond with. This is because it contains a nitrogen atom (n), which is one of the three atoms (the others being. Nh3 (ammonia) does have hydrogen bonding. Ammonia molecules joined together by hydrogen bonds makeup ammonia. The nitrogen atom in ammonia has a lone pair of electrons, which can form a hydrogen bond with. A molecule of ammonia can give and receive up to three hydrogen bonds. Actually, nh3 is able to form hydrogen bonds with water and even with itself. Yes, ammonia (nh3) can form hydrogen bonds. Yes, ammonia (nh3) can form hydrogen bonds.
Ammonia molecules joined together by hydrogen bonds makeup ammonia. The nitrogen atom in ammonia has a lone pair of electrons, which can form a hydrogen bond with. However, forming hydrogen bonds is an. Yes, ammonia (nh3) can form hydrogen bonds. Yes, ammonia (nh3) can form hydrogen bonds. This is because it contains a nitrogen atom (n), which is one of the three atoms (the others being. The nitrogen atom in ammonia has a lone pair of electrons, which can form a hydrogen bond with. Actually, nh3 is able to form hydrogen bonds with water and even with itself. Nh3 (ammonia) does have hydrogen bonding. A molecule of ammonia can give and receive up to three hydrogen bonds.
The nitrogen atom in ammonia has a lone pair of electrons, which can form a hydrogen bond with. Actually, nh3 is able to form hydrogen bonds with water and even with itself. Nh3 (ammonia) does have hydrogen bonding. Yes, ammonia (nh3) can form hydrogen bonds. Ammonia molecules joined together by hydrogen bonds makeup ammonia. However, forming hydrogen bonds is an. The nitrogen atom in ammonia has a lone pair of electrons, which can form a hydrogen bond with. Yes, ammonia (nh3) can form hydrogen bonds. This is because it contains a nitrogen atom (n), which is one of the three atoms (the others being. A molecule of ammonia can give and receive up to three hydrogen bonds.
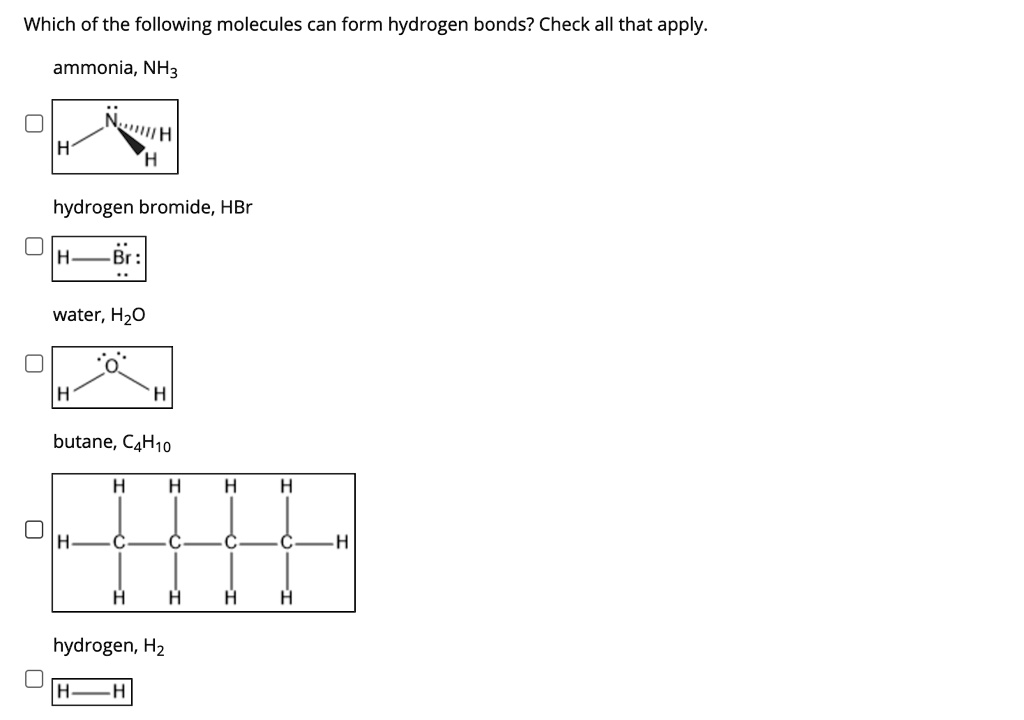
SOLVED Which of the following molecules can form hydrogen bonds? Check
A molecule of ammonia can give and receive up to three hydrogen bonds. Actually, nh3 is able to form hydrogen bonds with water and even with itself. The nitrogen atom in ammonia has a lone pair of electrons, which can form a hydrogen bond with. Nh3 (ammonia) does have hydrogen bonding. However, forming hydrogen bonds is an.
SOLVED Which of these molecules can form hydrogen bonds between them
However, forming hydrogen bonds is an. The nitrogen atom in ammonia has a lone pair of electrons, which can form a hydrogen bond with. Ammonia molecules joined together by hydrogen bonds makeup ammonia. A molecule of ammonia can give and receive up to three hydrogen bonds. Actually, nh3 is able to form hydrogen bonds with water and even with itself.
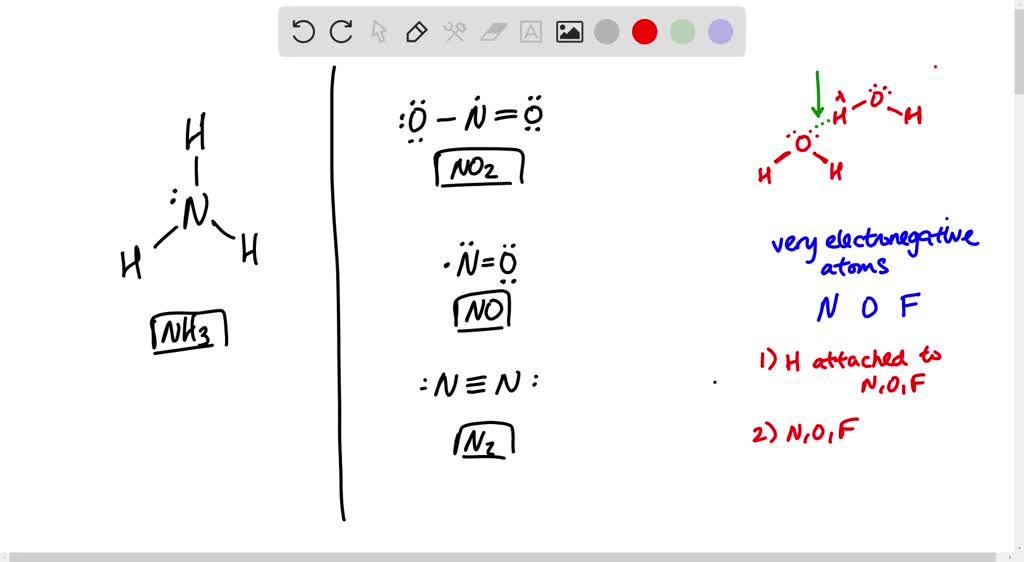
SOLVED Can NH3 form hydrogen bonds with NO2 or NO or N2?
Actually, nh3 is able to form hydrogen bonds with water and even with itself. The nitrogen atom in ammonia has a lone pair of electrons, which can form a hydrogen bond with. Yes, ammonia (nh3) can form hydrogen bonds. This is because it contains a nitrogen atom (n), which is one of the three atoms (the others being. Ammonia molecules.
Types of Chemical Bonds
Yes, ammonia (nh3) can form hydrogen bonds. Actually, nh3 is able to form hydrogen bonds with water and even with itself. Ammonia molecules joined together by hydrogen bonds makeup ammonia. The nitrogen atom in ammonia has a lone pair of electrons, which can form a hydrogen bond with. Nh3 (ammonia) does have hydrogen bonding.
hydrogen bond. chemistry lesson. Infographic. hydrogen bonds between
A molecule of ammonia can give and receive up to three hydrogen bonds. However, forming hydrogen bonds is an. The nitrogen atom in ammonia has a lone pair of electrons, which can form a hydrogen bond with. This is because it contains a nitrogen atom (n), which is one of the three atoms (the others being. Actually, nh3 is able.
Why can’t NH3 have 4 Hydrogen bonds like H2O? r/chemhelp
This is because it contains a nitrogen atom (n), which is one of the three atoms (the others being. A molecule of ammonia can give and receive up to three hydrogen bonds. However, forming hydrogen bonds is an. The nitrogen atom in ammonia has a lone pair of electrons, which can form a hydrogen bond with. The nitrogen atom in.
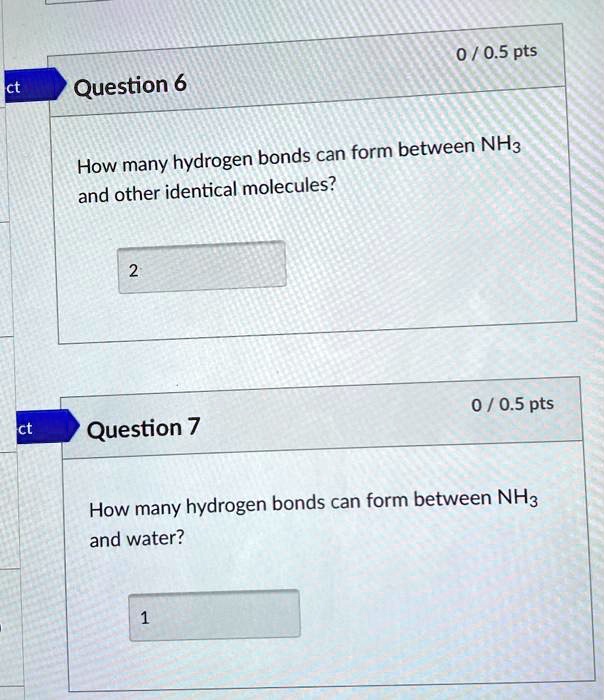
SOLVED 0 / 0.5 pts Question 6 How many hydrogen bonds can form between
Yes, ammonia (nh3) can form hydrogen bonds. The nitrogen atom in ammonia has a lone pair of electrons, which can form a hydrogen bond with. The nitrogen atom in ammonia has a lone pair of electrons, which can form a hydrogen bond with. Actually, nh3 is able to form hydrogen bonds with water and even with itself. Nh3 (ammonia) does.
Hydrogen Bonding
Yes, ammonia (nh3) can form hydrogen bonds. Yes, ammonia (nh3) can form hydrogen bonds. A molecule of ammonia can give and receive up to three hydrogen bonds. The nitrogen atom in ammonia has a lone pair of electrons, which can form a hydrogen bond with. Actually, nh3 is able to form hydrogen bonds with water and even with itself.
Nh3 H2o Estudiar
Nh3 (ammonia) does have hydrogen bonding. Yes, ammonia (nh3) can form hydrogen bonds. The nitrogen atom in ammonia has a lone pair of electrons, which can form a hydrogen bond with. Ammonia molecules joined together by hydrogen bonds makeup ammonia. However, forming hydrogen bonds is an.
[Solved] Ammonia, NH3, exhibits hydrogen bonding. Using five molecules
This is because it contains a nitrogen atom (n), which is one of the three atoms (the others being. Yes, ammonia (nh3) can form hydrogen bonds. The nitrogen atom in ammonia has a lone pair of electrons, which can form a hydrogen bond with. The nitrogen atom in ammonia has a lone pair of electrons, which can form a hydrogen.
The Nitrogen Atom In Ammonia Has A Lone Pair Of Electrons, Which Can Form A Hydrogen Bond With.
A molecule of ammonia can give and receive up to three hydrogen bonds. Yes, ammonia (nh3) can form hydrogen bonds. Actually, nh3 is able to form hydrogen bonds with water and even with itself. Nh3 (ammonia) does have hydrogen bonding.
However, Forming Hydrogen Bonds Is An.
Yes, ammonia (nh3) can form hydrogen bonds. Ammonia molecules joined together by hydrogen bonds makeup ammonia. The nitrogen atom in ammonia has a lone pair of electrons, which can form a hydrogen bond with. This is because it contains a nitrogen atom (n), which is one of the three atoms (the others being.