Enthalpy Khan Academy
Enthalpy Khan Academy - Khan academy is a 501 (c) (3) nonprofit organization. Khan academy is a 501 (c) (3) nonprofit organization. As an example of a reaction, let's look. Therefore the change in enthalpy for the reaction is negative and this is called an exothermic reaction.
Khan academy is a 501 (c) (3) nonprofit organization. Therefore the change in enthalpy for the reaction is negative and this is called an exothermic reaction. Khan academy is a 501 (c) (3) nonprofit organization. As an example of a reaction, let's look.
Khan academy is a 501 (c) (3) nonprofit organization. As an example of a reaction, let's look. Therefore the change in enthalpy for the reaction is negative and this is called an exothermic reaction. Khan academy is a 501 (c) (3) nonprofit organization.
Standard enthalpy of atomisation Thermodynamics Chemistry Khan
Khan academy is a 501 (c) (3) nonprofit organization. Khan academy is a 501 (c) (3) nonprofit organization. As an example of a reaction, let's look. Therefore the change in enthalpy for the reaction is negative and this is called an exothermic reaction.
How To Pronounce Enthalpy Pronunciation Academy YouTube
Therefore the change in enthalpy for the reaction is negative and this is called an exothermic reaction. As an example of a reaction, let's look. Khan academy is a 501 (c) (3) nonprofit organization. Khan academy is a 501 (c) (3) nonprofit organization.
Bond enthalpy and enthalpy of reaction Chemistry Khan Academy Urdu
Therefore the change in enthalpy for the reaction is negative and this is called an exothermic reaction. Khan academy is a 501 (c) (3) nonprofit organization. As an example of a reaction, let's look. Khan academy is a 501 (c) (3) nonprofit organization.
Enthalpy Thermodynamics Chemistry Khan Academy YouTube
Khan academy is a 501 (c) (3) nonprofit organization. As an example of a reaction, let's look. Khan academy is a 501 (c) (3) nonprofit organization. Therefore the change in enthalpy for the reaction is negative and this is called an exothermic reaction.
Khan Academy Enthalpy Instructional Video for 9th 10th Grade
Khan academy is a 501 (c) (3) nonprofit organization. As an example of a reaction, let's look. Khan academy is a 501 (c) (3) nonprofit organization. Therefore the change in enthalpy for the reaction is negative and this is called an exothermic reaction.
Hess's law and reaction enthalpy change Chemistry Khan Academy
Therefore the change in enthalpy for the reaction is negative and this is called an exothermic reaction. Khan academy is a 501 (c) (3) nonprofit organization. As an example of a reaction, let's look. Khan academy is a 501 (c) (3) nonprofit organization.
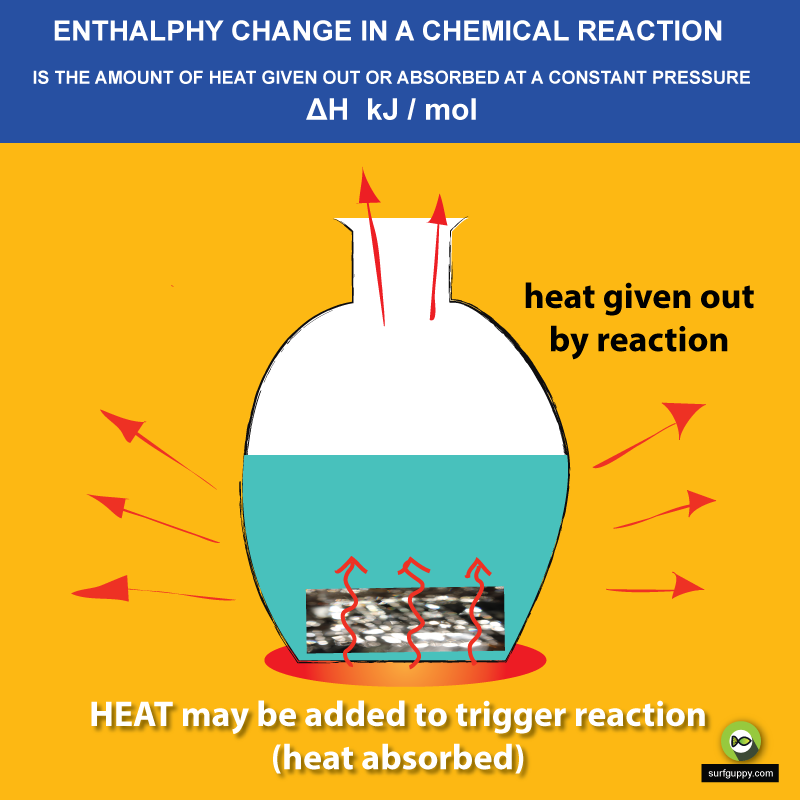
Enthalpy Surfguppy Chemistry made easy visual learning
Therefore the change in enthalpy for the reaction is negative and this is called an exothermic reaction. Khan academy is a 501 (c) (3) nonprofit organization. As an example of a reaction, let's look. Khan academy is a 501 (c) (3) nonprofit organization.
Enthalpy of reaction Thermodynamics AP Chemistry Khan Academy
Khan academy is a 501 (c) (3) nonprofit organization. Therefore the change in enthalpy for the reaction is negative and this is called an exothermic reaction. As an example of a reaction, let's look. Khan academy is a 501 (c) (3) nonprofit organization.
Lattice Enthalpy Thermodynamics Chemistry Khan Academy YouTube
Khan academy is a 501 (c) (3) nonprofit organization. As an example of a reaction, let's look. Khan academy is a 501 (c) (3) nonprofit organization. Therefore the change in enthalpy for the reaction is negative and this is called an exothermic reaction.
ALIGNMENT OF MOE SYLLABUS WITH KHAN ACADEMY RESOURCES FOR CHEMISTRY
Khan academy is a 501 (c) (3) nonprofit organization. As an example of a reaction, let's look. Therefore the change in enthalpy for the reaction is negative and this is called an exothermic reaction. Khan academy is a 501 (c) (3) nonprofit organization.
Khan Academy Is A 501 (C) (3) Nonprofit Organization.
As an example of a reaction, let's look. Khan academy is a 501 (c) (3) nonprofit organization. Therefore the change in enthalpy for the reaction is negative and this is called an exothermic reaction.