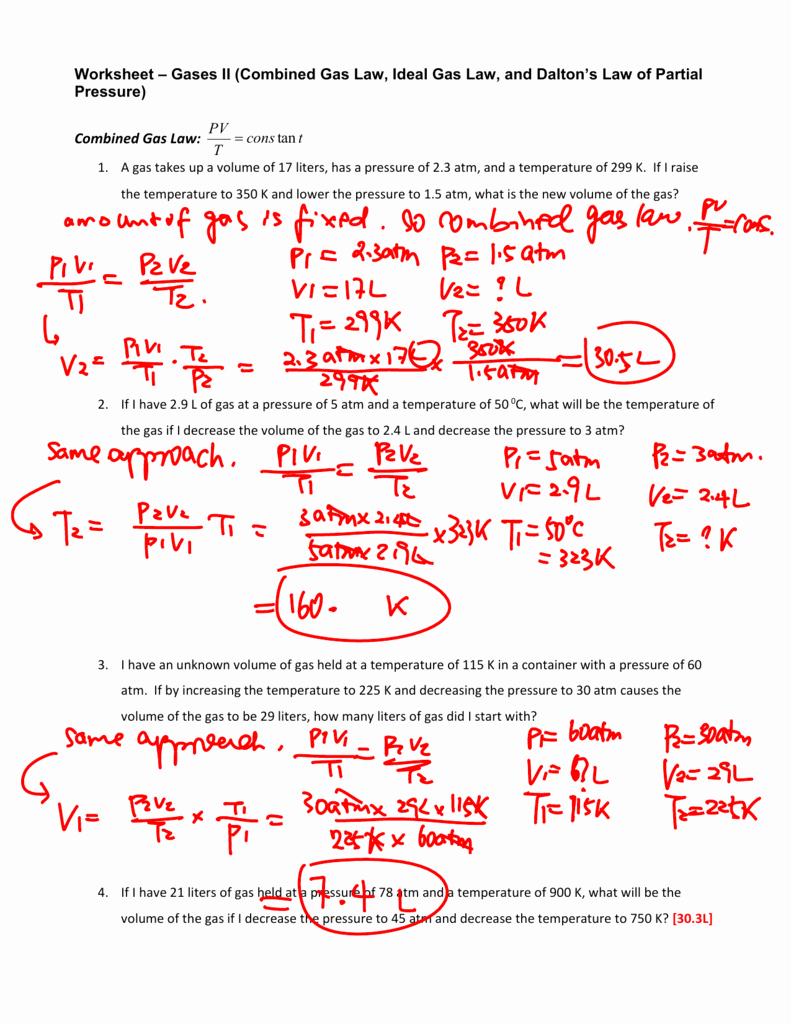
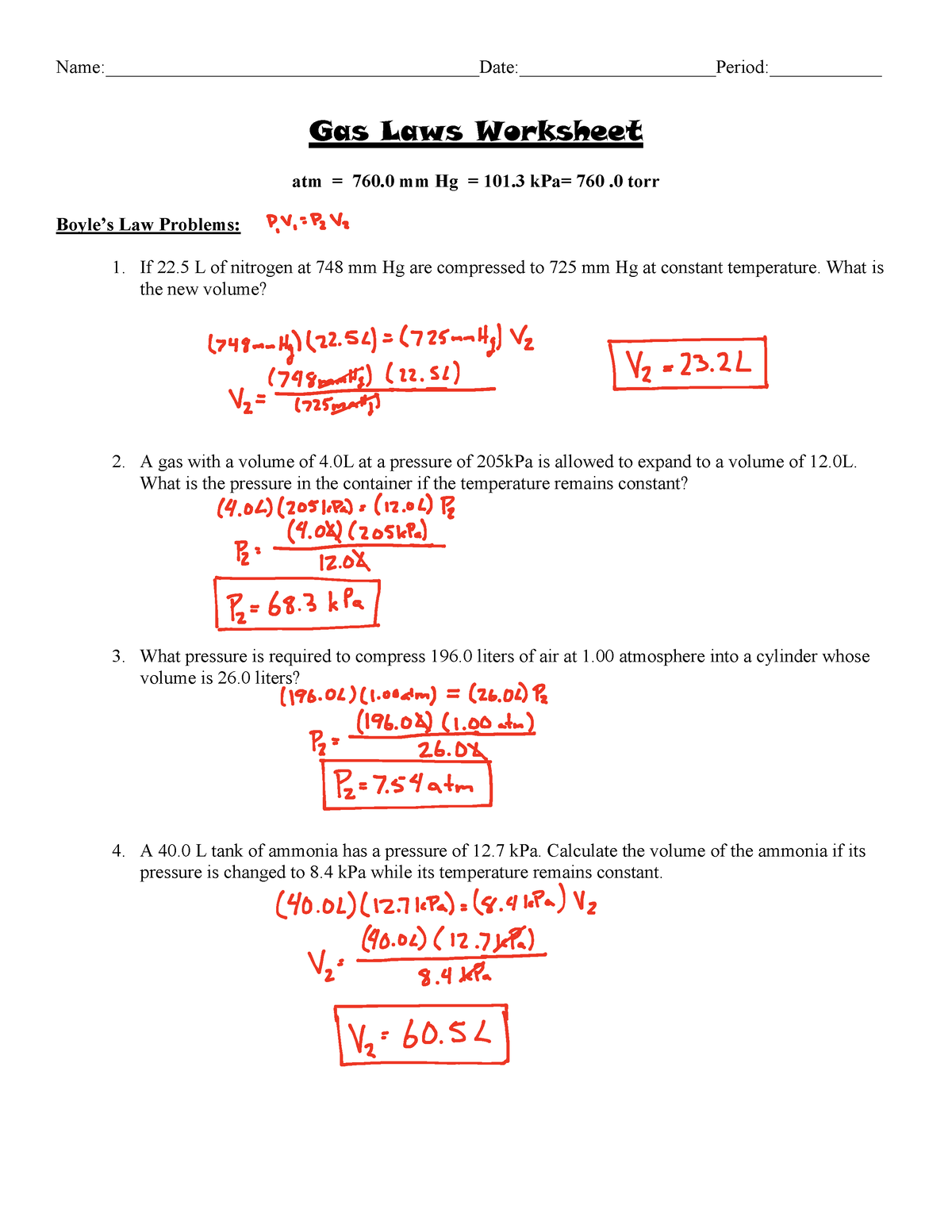
Ideal Gas Law Worksheet Answers
Ideal Gas Law Worksheet Answers - The gas laws worksheet focuses on several fundamental gas laws, such as boyle’s law, charles’s law, avogadro’s law, and the ideal. The ideal gas law directions: 2) at what temperature would 2.10 moles of n2 gas have a. Show your work, including proper units, to earn full credit. 1) given the following sets of values, calculate the unknown quantity. Solve each of the following problems. How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9 l? The ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the volume of the gas, n is the number of moles of gas present, r is the. Assume that the lungs are at.
Solve each of the following problems. The ideal gas law directions: Show your work, including proper units, to earn full credit. Assume that the lungs are at. How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9 l? The gas laws worksheet focuses on several fundamental gas laws, such as boyle’s law, charles’s law, avogadro’s law, and the ideal. The ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the volume of the gas, n is the number of moles of gas present, r is the. 2) at what temperature would 2.10 moles of n2 gas have a. 1) given the following sets of values, calculate the unknown quantity.
Solve each of the following problems. 2) at what temperature would 2.10 moles of n2 gas have a. The gas laws worksheet focuses on several fundamental gas laws, such as boyle’s law, charles’s law, avogadro’s law, and the ideal. 1) given the following sets of values, calculate the unknown quantity. Show your work, including proper units, to earn full credit. The ideal gas law directions: The ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the volume of the gas, n is the number of moles of gas present, r is the. Assume that the lungs are at. How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9 l?
Ideal Gas Law Problems Worksheet Worksheets For Kindergarten
The ideal gas law directions: Show your work, including proper units, to earn full credit. 1) given the following sets of values, calculate the unknown quantity. Assume that the lungs are at. The ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the volume of the gas, n is the number of moles.
Ideal Gas Law Worksheet Answers
2) at what temperature would 2.10 moles of n2 gas have a. Assume that the lungs are at. How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9 l? Show your work, including proper units, to earn full credit. The ideal gas law directions:
Ideal Gas Law Worksheet PV = nRT
Solve each of the following problems. The gas laws worksheet focuses on several fundamental gas laws, such as boyle’s law, charles’s law, avogadro’s law, and the ideal. Show your work, including proper units, to earn full credit. The ideal gas law directions: The ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the.
Ideal Gas Law Worksheet 2 Answer Ideal Gas Law Worksheet PV = nRT Use
The gas laws worksheet focuses on several fundamental gas laws, such as boyle’s law, charles’s law, avogadro’s law, and the ideal. Assume that the lungs are at. The ideal gas law directions: Show your work, including proper units, to earn full credit. 2) at what temperature would 2.10 moles of n2 gas have a.
Ideal Gas Law Practice With Answers
2) at what temperature would 2.10 moles of n2 gas have a. The ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the volume of the gas, n is the number of moles of gas present, r is the. Assume that the lungs are at. The gas laws worksheet focuses on several fundamental.
Ideal Gas Law Worksheet Science ShowMe
Solve each of the following problems. 2) at what temperature would 2.10 moles of n2 gas have a. Assume that the lungs are at. Show your work, including proper units, to earn full credit. The gas laws worksheet focuses on several fundamental gas laws, such as boyle’s law, charles’s law, avogadro’s law, and the ideal.
Gas Laws Worksheet answer key Gas Laws Worksheet atm = 760 mm Hg
The gas laws worksheet focuses on several fundamental gas laws, such as boyle’s law, charles’s law, avogadro’s law, and the ideal. 1) given the following sets of values, calculate the unknown quantity. How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9 l? Show your work, including proper units, to earn.
Ideal Gas Law Questions And Answers
How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9 l? The ideal gas law directions: 2) at what temperature would 2.10 moles of n2 gas have a. Assume that the lungs are at. The gas laws worksheet focuses on several fundamental gas laws, such as boyle’s law, charles’s law, avogadro’s.
Ideal Gas Law Worksheet 144 Answer Key Greenus
Show your work, including proper units, to earn full credit. 2) at what temperature would 2.10 moles of n2 gas have a. The ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the volume of the gas, n is the number of moles of gas present, r is the. Solve each of the.
SOLUTION Ideal gass law worksheet Studypool
Assume that the lungs are at. The ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the volume of the gas, n is the number of moles of gas present, r is the. The gas laws worksheet focuses on several fundamental gas laws, such as boyle’s law, charles’s law, avogadro’s law, and the.
1) Given The Following Sets Of Values, Calculate The Unknown Quantity.
Assume that the lungs are at. The ideal gas law directions: Show your work, including proper units, to earn full credit. Solve each of the following problems.
2) At What Temperature Would 2.10 Moles Of N2 Gas Have A.
How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9 l? The gas laws worksheet focuses on several fundamental gas laws, such as boyle’s law, charles’s law, avogadro’s law, and the ideal. The ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the volume of the gas, n is the number of moles of gas present, r is the.