Khan Academy Electron Configuration
Khan Academy Electron Configuration - Electron configurations describe where electrons are located around the nucleus of an atom. For example, the electron configuration of lithium,. If you're seeing this message, it means we're having trouble loading external resources on our website. If you're behind a web filter, please. Writes out the quantum numbers for the electrons in h and he. Test your knowledge of the skills in this course. Counting protons, electrons, and neutronsget 5 of 7 questions to level up!. Introduces aufbau principle, pauli exclusion principle, and orbital notation.
If you're behind a web filter, please. Test your knowledge of the skills in this course. If you're seeing this message, it means we're having trouble loading external resources on our website. Introduces aufbau principle, pauli exclusion principle, and orbital notation. Writes out the quantum numbers for the electrons in h and he. For example, the electron configuration of lithium,. Electron configurations describe where electrons are located around the nucleus of an atom. Counting protons, electrons, and neutronsget 5 of 7 questions to level up!.
Electron configurations describe where electrons are located around the nucleus of an atom. If you're behind a web filter, please. For example, the electron configuration of lithium,. Writes out the quantum numbers for the electrons in h and he. Test your knowledge of the skills in this course. If you're seeing this message, it means we're having trouble loading external resources on our website. Counting protons, electrons, and neutronsget 5 of 7 questions to level up!. Introduces aufbau principle, pauli exclusion principle, and orbital notation.
Electron configurations for the first period Chemistry Khan Academy
Electron configurations describe where electrons are located around the nucleus of an atom. Introduces aufbau principle, pauli exclusion principle, and orbital notation. Writes out the quantum numbers for the electrons in h and he. Counting protons, electrons, and neutronsget 5 of 7 questions to level up!. If you're behind a web filter, please.
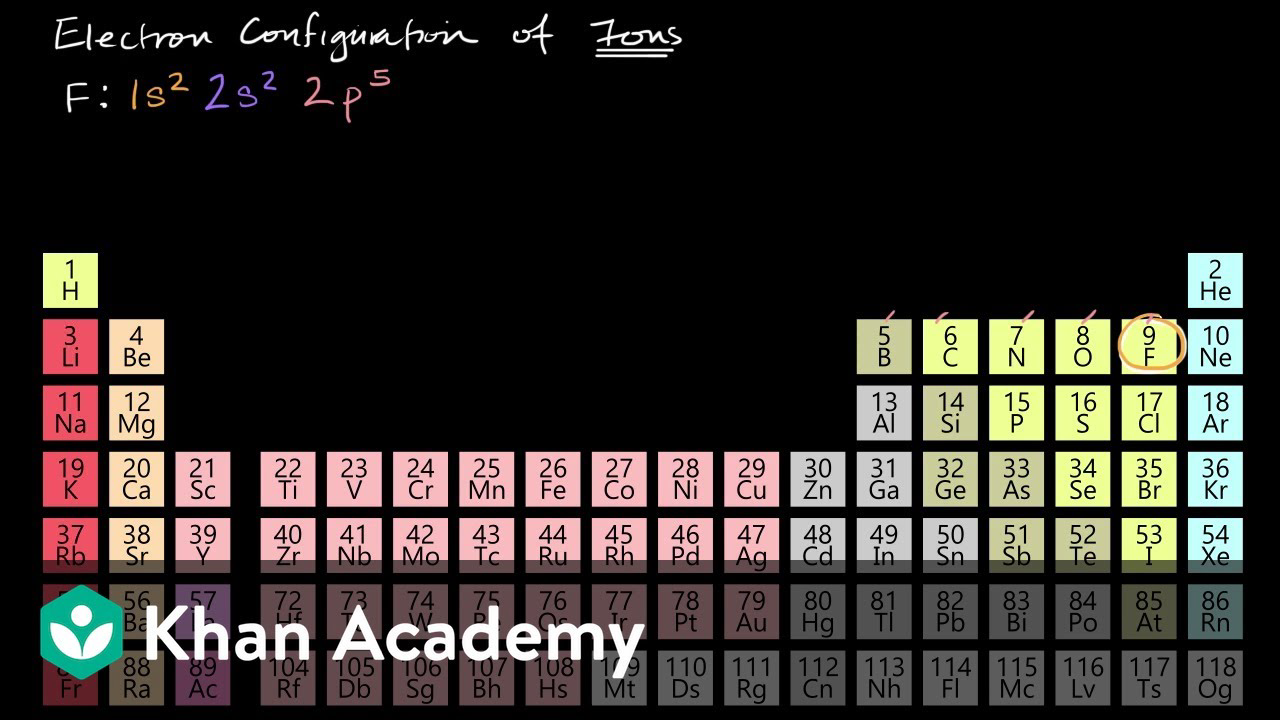
Electron configurations of ions Atomic structure and properties AP
For example, the electron configuration of lithium,. If you're seeing this message, it means we're having trouble loading external resources on our website. Writes out the quantum numbers for the electrons in h and he. Counting protons, electrons, and neutronsget 5 of 7 questions to level up!. Test your knowledge of the skills in this course.
The periodic table, electron shells, and orbitals (article) Khan
If you're behind a web filter, please. Writes out the quantum numbers for the electrons in h and he. For example, the electron configuration of lithium,. Counting protons, electrons, and neutronsget 5 of 7 questions to level up!. Test your knowledge of the skills in this course.
Valence electrons and bonding Chemistry of life Biology Khan
Electron configurations describe where electrons are located around the nucleus of an atom. Counting protons, electrons, and neutronsget 5 of 7 questions to level up!. If you're seeing this message, it means we're having trouble loading external resources on our website. Introduces aufbau principle, pauli exclusion principle, and orbital notation. For example, the electron configuration of lithium,.
Apply electron configurations (practice) Khan Academy
Writes out the quantum numbers for the electrons in h and he. Test your knowledge of the skills in this course. Electron configurations describe where electrons are located around the nucleus of an atom. Introduces aufbau principle, pauli exclusion principle, and orbital notation. If you're behind a web filter, please.
More on orbitals and electron configuration Chemistry Khan Academy
If you're behind a web filter, please. Test your knowledge of the skills in this course. Writes out the quantum numbers for the electrons in h and he. Electron configurations describe where electrons are located around the nucleus of an atom. Introduces aufbau principle, pauli exclusion principle, and orbital notation.
Khan Academy Khan academy, Chemistry, Electrons
Writes out the quantum numbers for the electrons in h and he. If you're behind a web filter, please. If you're seeing this message, it means we're having trouble loading external resources on our website. For example, the electron configuration of lithium,. Counting protons, electrons, and neutronsget 5 of 7 questions to level up!.
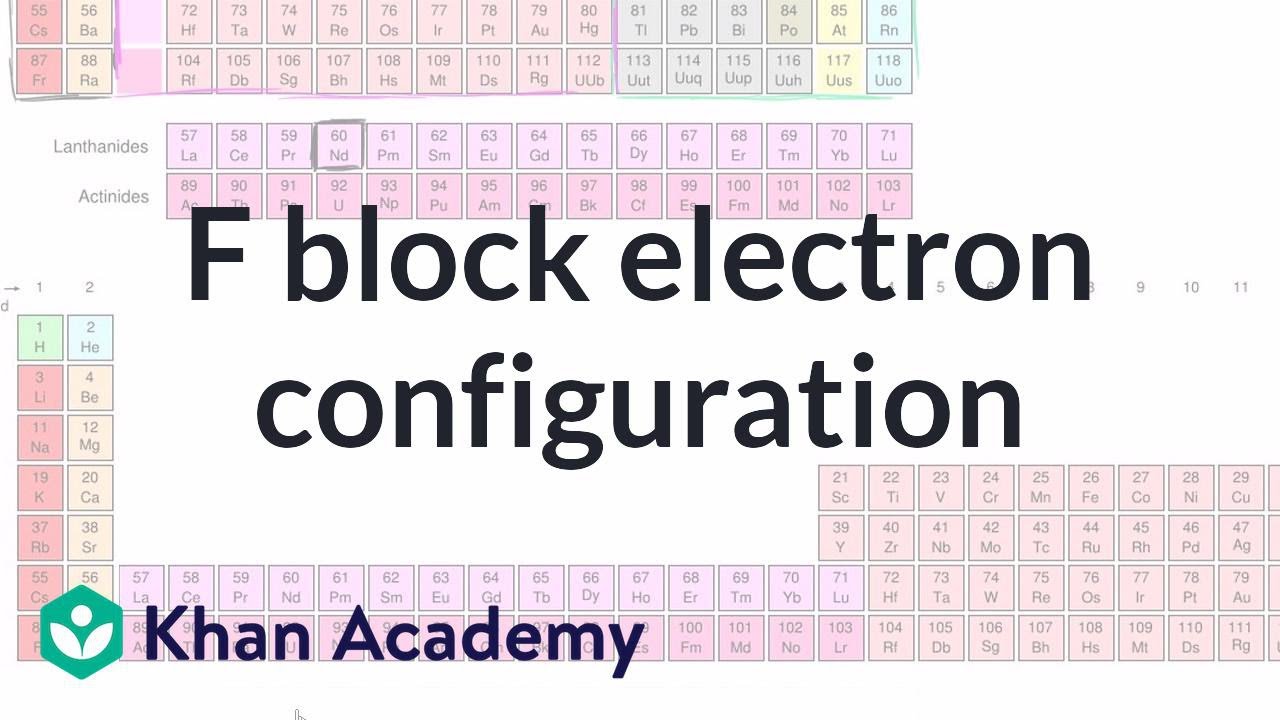
Electron configuration for f block element Nd Chemistry Khan
Test your knowledge of the skills in this course. Electron configurations describe where electrons are located around the nucleus of an atom. For example, the electron configuration of lithium,. Writes out the quantum numbers for the electrons in h and he. If you're behind a web filter, please.
Electron configuration for d block element Chemistry Khan Academy YouTube
Writes out the quantum numbers for the electrons in h and he. If you're behind a web filter, please. Introduces aufbau principle, pauli exclusion principle, and orbital notation. Counting protons, electrons, and neutronsget 5 of 7 questions to level up!. Test your knowledge of the skills in this course.
Electron configurations for the third and fourth periods Chemistry
Counting protons, electrons, and neutronsget 5 of 7 questions to level up!. For example, the electron configuration of lithium,. Electron configurations describe where electrons are located around the nucleus of an atom. Writes out the quantum numbers for the electrons in h and he. If you're behind a web filter, please.
If You're Behind A Web Filter, Please.
Test your knowledge of the skills in this course. Electron configurations describe where electrons are located around the nucleus of an atom. Introduces aufbau principle, pauli exclusion principle, and orbital notation. If you're seeing this message, it means we're having trouble loading external resources on our website.
Counting Protons, Electrons, And Neutronsget 5 Of 7 Questions To Level Up!.
For example, the electron configuration of lithium,. Writes out the quantum numbers for the electrons in h and he.