Magnesium And Oxygen Combine To Form Which Ionic Compound
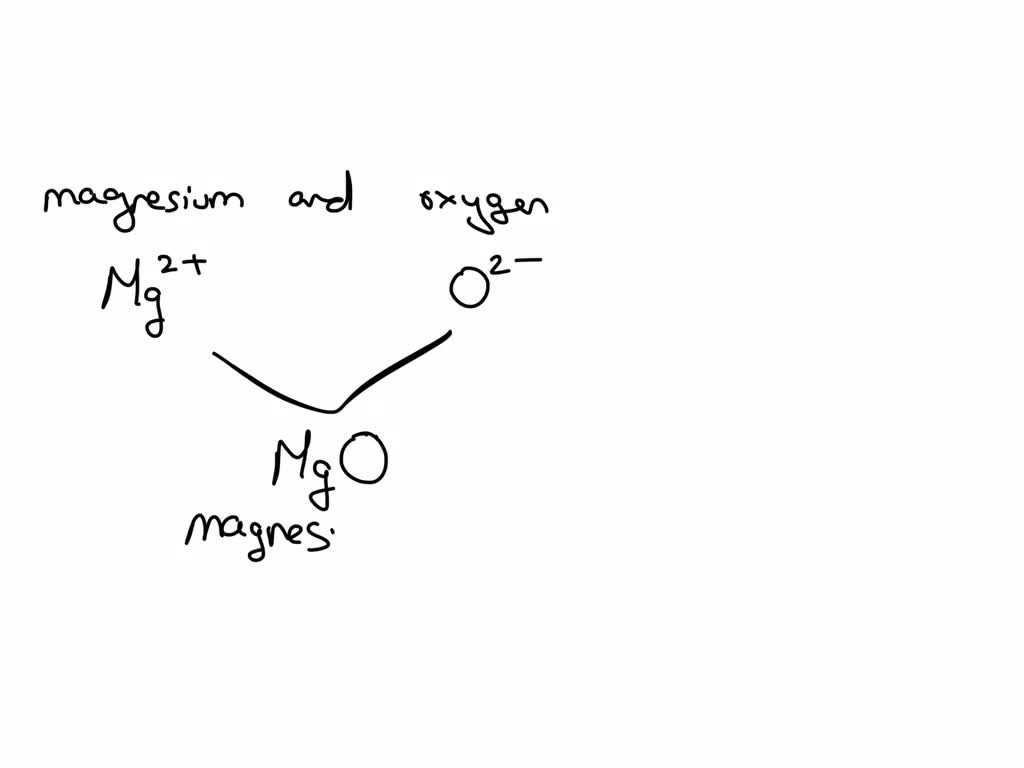
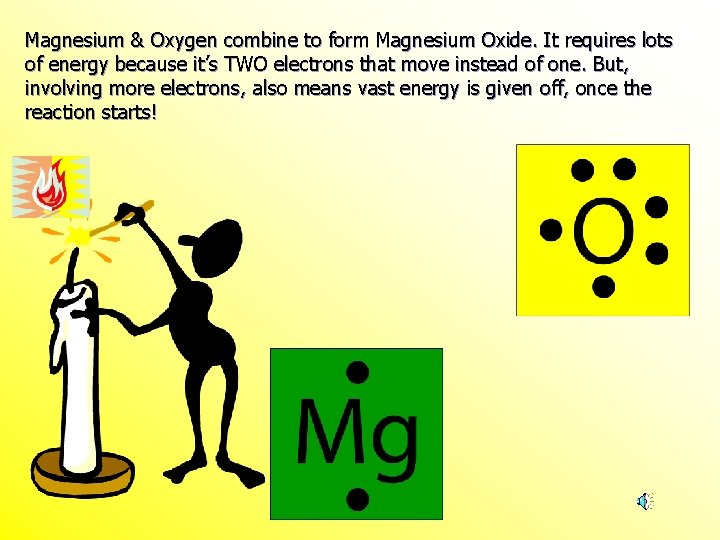
Magnesium And Oxygen Combine To Form Which Ionic Compound - Magnesium, a metal, gives up two electrons to. When the reaction with oxygen occurs, these two electrons are donated by the magnesium, forming positively charged mg 2+ ions. The ionic compound that forms from magnesium and oxygen is magnesium oxide, which has the formula mgo. Yes, oxygen and magnesium form an ionic compound called magnesium oxide. Magnesium has a charge of 2 +, while oxygen has a charge of 2 −. This occurs when magnesium loses. The burning of magnesium metal reacts with oxygen found in the. Magnesium and oxygen combine to form magnesium oxide, represented by the chemical formula mgo. This is because magnesium (mg) loses 2. The correct formula for the ionic compound produced by the reaction of magnesium metal with oxygen gas is mgo(s).
The ionic compound that forms from magnesium and oxygen is magnesium oxide, which has the formula mgo. Magnesium has a charge of 2 +, while oxygen has a charge of 2 −. The correct formula for the ionic compound produced by the reaction of magnesium metal with oxygen gas is mgo(s). This occurs when magnesium loses. Magnesium, a metal, gives up two electrons to. Yes, oxygen and magnesium form an ionic compound called magnesium oxide. The burning of magnesium metal reacts with oxygen found in the. This is because magnesium (mg) loses 2. Magnesium and oxygen combine to form magnesium oxide, represented by the chemical formula mgo. When the reaction with oxygen occurs, these two electrons are donated by the magnesium, forming positively charged mg 2+ ions.
Magnesium, a metal, gives up two electrons to. Magnesium and oxygen combine to form magnesium oxide, represented by the chemical formula mgo. Magnesium has a charge of 2 +, while oxygen has a charge of 2 −. The ionic compound that forms from magnesium and oxygen is magnesium oxide, which has the formula mgo. The burning of magnesium metal reacts with oxygen found in the. Yes, oxygen and magnesium form an ionic compound called magnesium oxide. This is because magnesium (mg) loses 2. When the reaction with oxygen occurs, these two electrons are donated by the magnesium, forming positively charged mg 2+ ions. The correct formula for the ionic compound produced by the reaction of magnesium metal with oxygen gas is mgo(s). This occurs when magnesium loses.
Which diagram shows the correct way to represent an ionic compound of
When the reaction with oxygen occurs, these two electrons are donated by the magnesium, forming positively charged mg 2+ ions. The correct formula for the ionic compound produced by the reaction of magnesium metal with oxygen gas is mgo(s). Magnesium has a charge of 2 +, while oxygen has a charge of 2 −. This is because magnesium (mg) loses.
magnesium oxygen reaction
This is because magnesium (mg) loses 2. The ionic compound that forms from magnesium and oxygen is magnesium oxide, which has the formula mgo. Magnesium has a charge of 2 +, while oxygen has a charge of 2 −. When the reaction with oxygen occurs, these two electrons are donated by the magnesium, forming positively charged mg 2+ ions. The.
SOLVED What happens when the compound Mgo is formed? (5 points) Oxygen
Magnesium has a charge of 2 +, while oxygen has a charge of 2 −. The correct formula for the ionic compound produced by the reaction of magnesium metal with oxygen gas is mgo(s). Magnesium, a metal, gives up two electrons to. Magnesium and oxygen combine to form magnesium oxide, represented by the chemical formula mgo. The ionic compound that.
When magnesium makes an ionic bond with oxygen it loses two electrons
Magnesium, a metal, gives up two electrons to. The ionic compound that forms from magnesium and oxygen is magnesium oxide, which has the formula mgo. Magnesium and oxygen combine to form magnesium oxide, represented by the chemical formula mgo. This is because magnesium (mg) loses 2. This occurs when magnesium loses.
Making Molecules First Look at the Periodic Table
This is because magnesium (mg) loses 2. The burning of magnesium metal reacts with oxygen found in the. Magnesium has a charge of 2 +, while oxygen has a charge of 2 −. Magnesium and oxygen combine to form magnesium oxide, represented by the chemical formula mgo. The correct formula for the ionic compound produced by the reaction of magnesium.
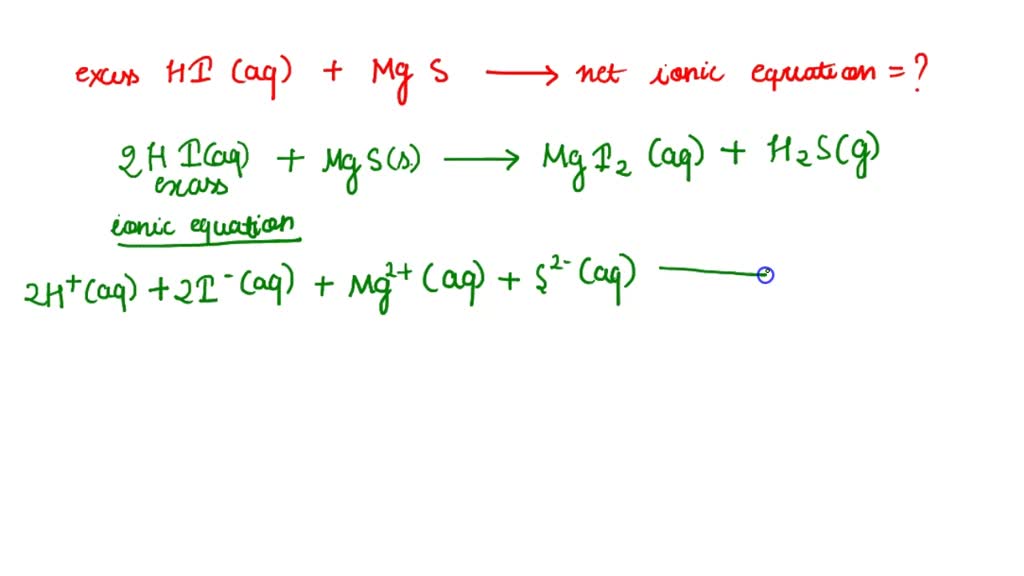
SOLVED Write net ionic equation for the reaction that occurs when
Magnesium and oxygen combine to form magnesium oxide, represented by the chemical formula mgo. Magnesium has a charge of 2 +, while oxygen has a charge of 2 −. The burning of magnesium metal reacts with oxygen found in the. Yes, oxygen and magnesium form an ionic compound called magnesium oxide. The correct formula for the ionic compound produced by.
Using Lewis Symbols Diagram the Reaction Between Magnesium and Oxygen
Yes, oxygen and magnesium form an ionic compound called magnesium oxide. This occurs when magnesium loses. Magnesium, a metal, gives up two electrons to. When the reaction with oxygen occurs, these two electrons are donated by the magnesium, forming positively charged mg 2+ ions. This is because magnesium (mg) loses 2.
Formation of magnesium oxide from magnesium and oxygen Brainly.in
Magnesium, a metal, gives up two electrons to. The correct formula for the ionic compound produced by the reaction of magnesium metal with oxygen gas is mgo(s). When the reaction with oxygen occurs, these two electrons are donated by the magnesium, forming positively charged mg 2+ ions. This occurs when magnesium loses. Magnesium and oxygen combine to form magnesium oxide,.
Bonding Basics
This is because magnesium (mg) loses 2. Magnesium, a metal, gives up two electrons to. Yes, oxygen and magnesium form an ionic compound called magnesium oxide. The correct formula for the ionic compound produced by the reaction of magnesium metal with oxygen gas is mgo(s). Magnesium has a charge of 2 +, while oxygen has a charge of 2 −.
Reaction Magnesium and Oxygen ThinkTac Science Experiment YouTube
Magnesium has a charge of 2 +, while oxygen has a charge of 2 −. Magnesium and oxygen combine to form magnesium oxide, represented by the chemical formula mgo. The burning of magnesium metal reacts with oxygen found in the. Magnesium, a metal, gives up two electrons to. Yes, oxygen and magnesium form an ionic compound called magnesium oxide.
The Correct Formula For The Ionic Compound Produced By The Reaction Of Magnesium Metal With Oxygen Gas Is Mgo(S).
This is because magnesium (mg) loses 2. The ionic compound that forms from magnesium and oxygen is magnesium oxide, which has the formula mgo. Magnesium has a charge of 2 +, while oxygen has a charge of 2 −. This occurs when magnesium loses.
The Burning Of Magnesium Metal Reacts With Oxygen Found In The.
Magnesium, a metal, gives up two electrons to. Yes, oxygen and magnesium form an ionic compound called magnesium oxide. Magnesium and oxygen combine to form magnesium oxide, represented by the chemical formula mgo. When the reaction with oxygen occurs, these two electrons are donated by the magnesium, forming positively charged mg 2+ ions.