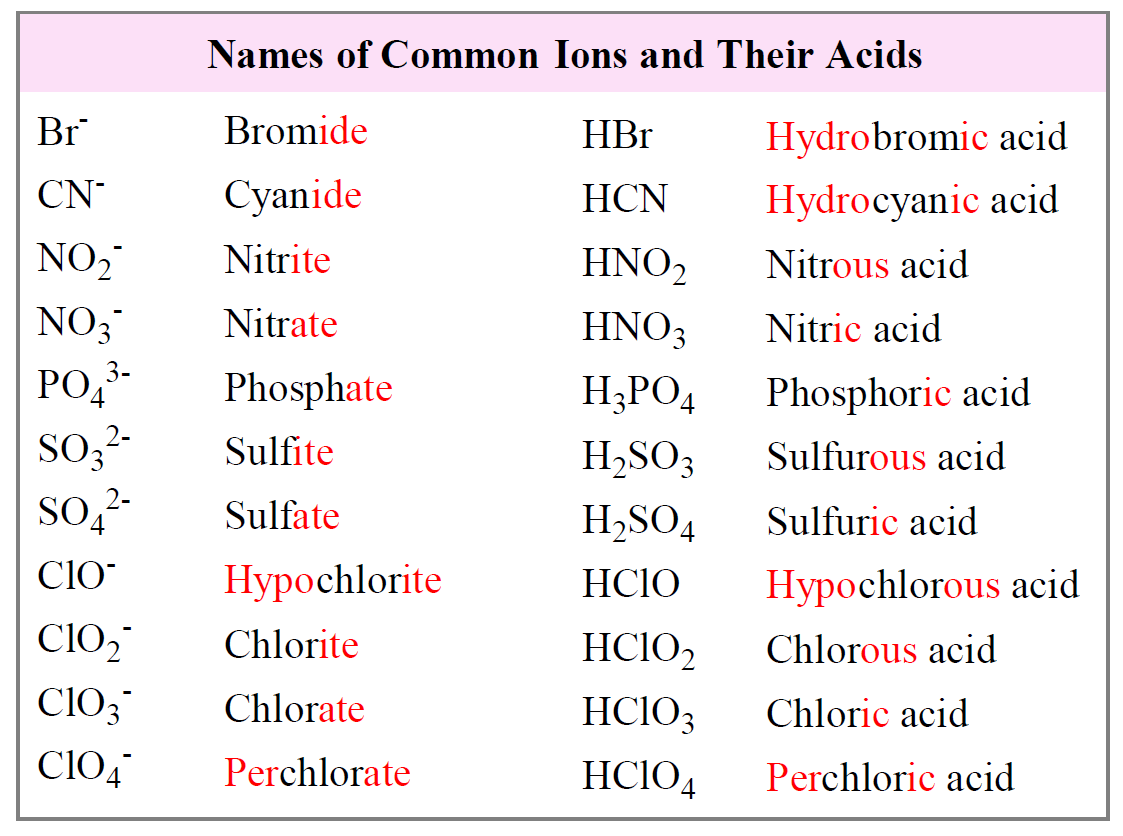
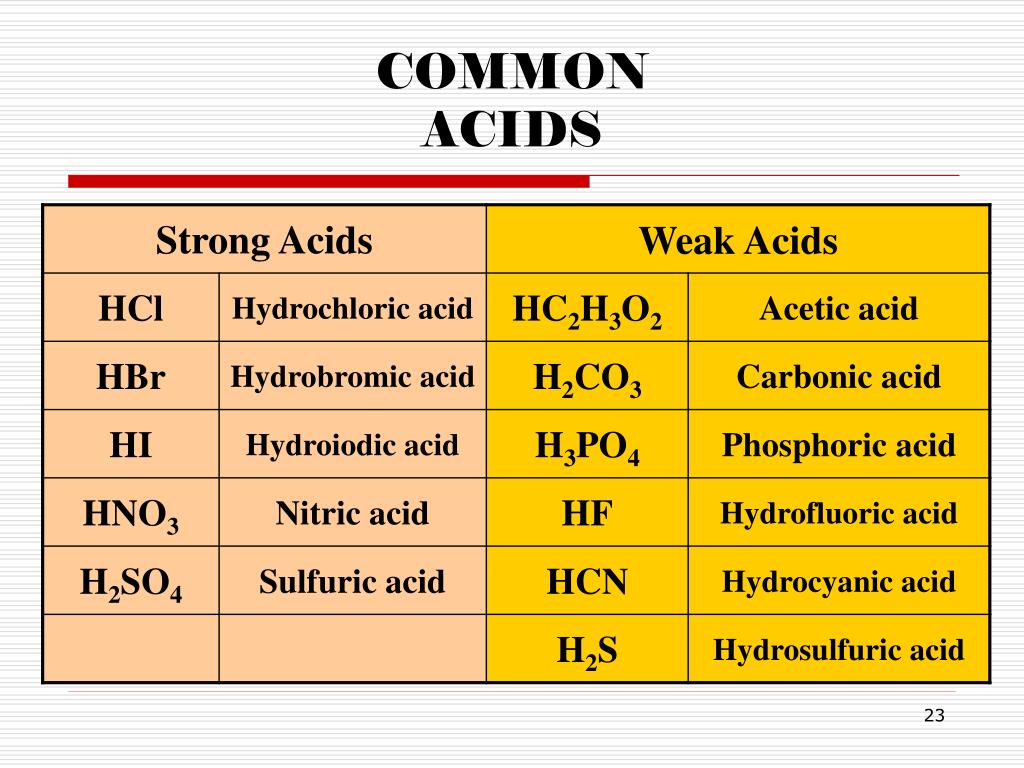
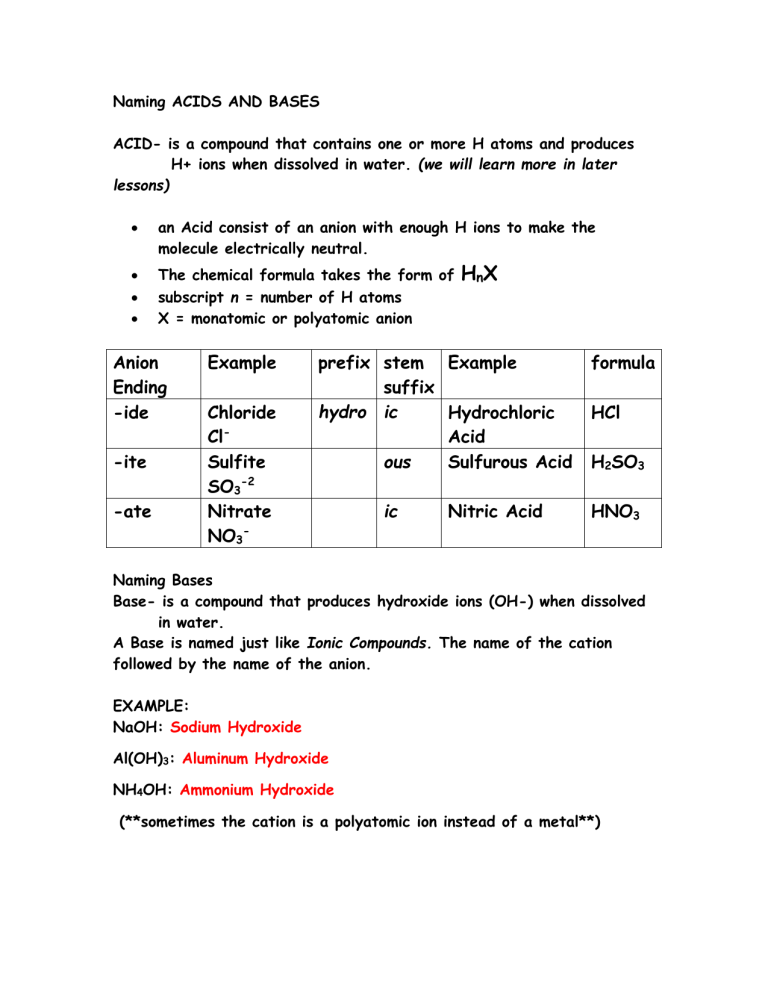
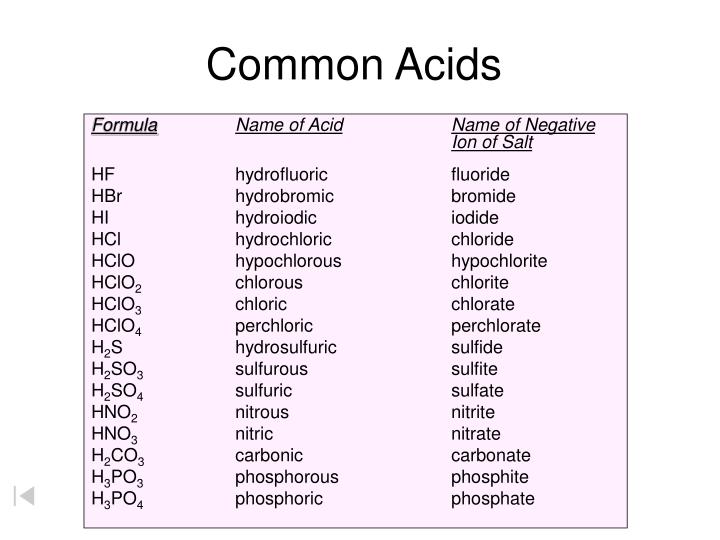
Naming Acids And Bases
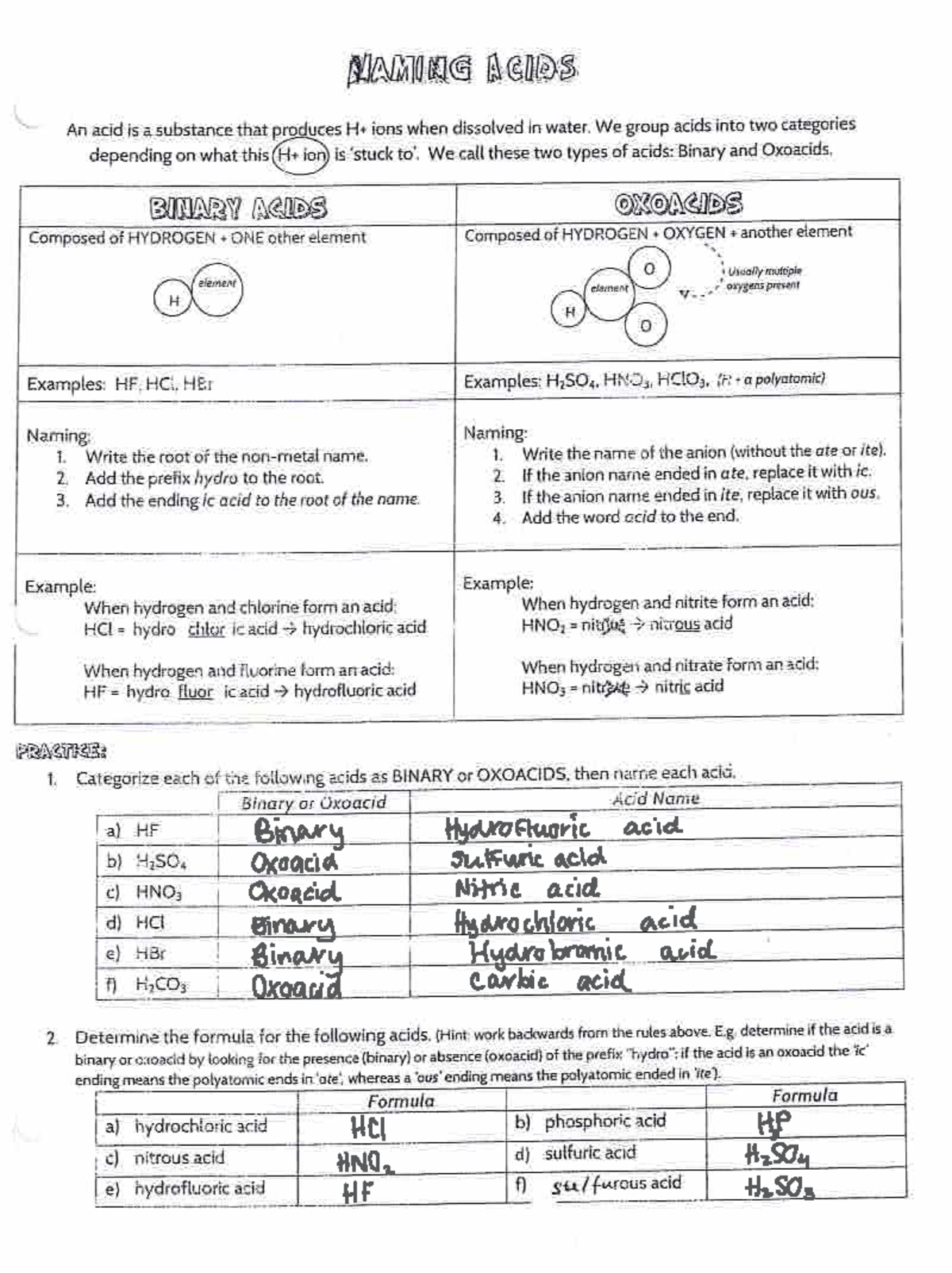
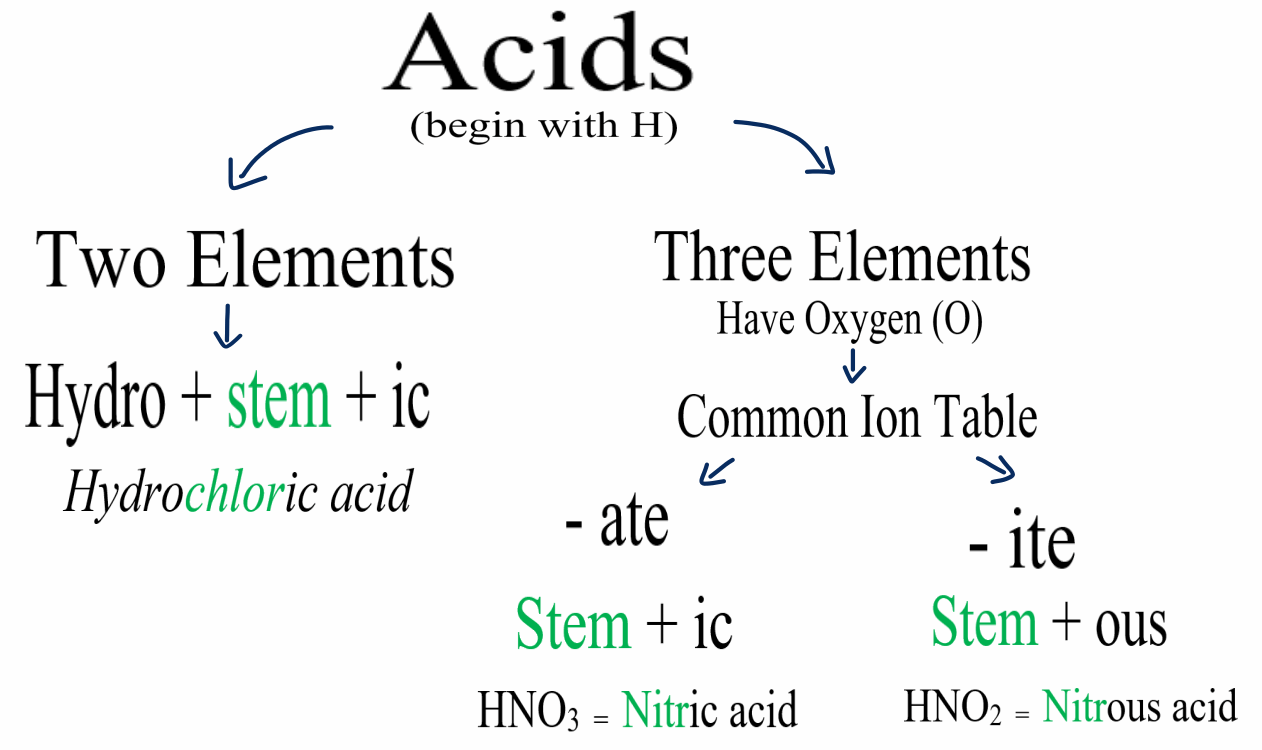
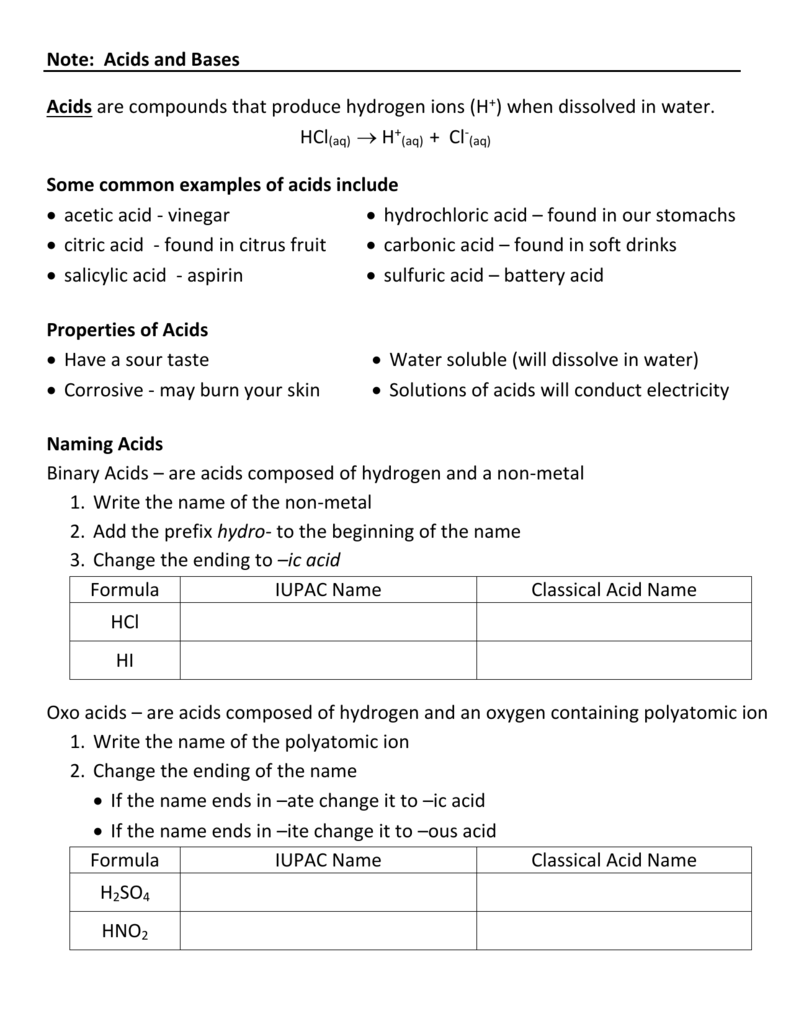
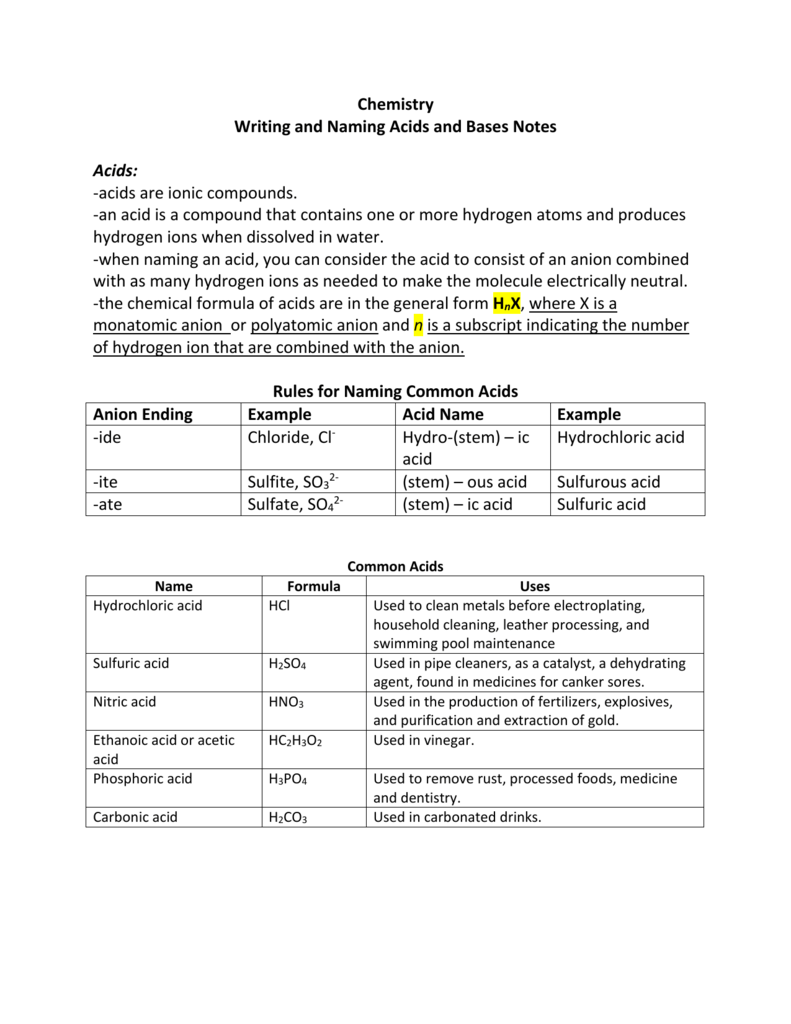
Naming Acids And Bases - The general properties of acids and bases are the following. More practice examples on nomenclature and formulas. To name a base, add the word hydroxide to the name of the cation: When naming acids and bases, remember that an acid always contains a hydrogen atom and an ion. An oxyacid is an acid that consists of hydrogen, oxygen, and a. Bases tend to follow the same rules as ionic compounds. In simple binary acids, one ion is attached to hydrogen.
To name a base, add the word hydroxide to the name of the cation: An oxyacid is an acid that consists of hydrogen, oxygen, and a. When naming acids and bases, remember that an acid always contains a hydrogen atom and an ion. Bases tend to follow the same rules as ionic compounds. In simple binary acids, one ion is attached to hydrogen. More practice examples on nomenclature and formulas. The general properties of acids and bases are the following.
When naming acids and bases, remember that an acid always contains a hydrogen atom and an ion. Bases tend to follow the same rules as ionic compounds. To name a base, add the word hydroxide to the name of the cation: An oxyacid is an acid that consists of hydrogen, oxygen, and a. The general properties of acids and bases are the following. In simple binary acids, one ion is attached to hydrogen. More practice examples on nomenclature and formulas.
Naming Acids wefwefwef Studocu
An oxyacid is an acid that consists of hydrogen, oxygen, and a. The general properties of acids and bases are the following. More practice examples on nomenclature and formulas. In simple binary acids, one ion is attached to hydrogen. To name a base, add the word hydroxide to the name of the cation:
Naming Acids & Bases
More practice examples on nomenclature and formulas. An oxyacid is an acid that consists of hydrogen, oxygen, and a. When naming acids and bases, remember that an acid always contains a hydrogen atom and an ion. To name a base, add the word hydroxide to the name of the cation: In simple binary acids, one ion is attached to hydrogen.
Naming Acids and Bases
To name a base, add the word hydroxide to the name of the cation: More practice examples on nomenclature and formulas. The general properties of acids and bases are the following. When naming acids and bases, remember that an acid always contains a hydrogen atom and an ion. Bases tend to follow the same rules as ionic compounds.
Naming Acids And Bases Practice
The general properties of acids and bases are the following. In simple binary acids, one ion is attached to hydrogen. Bases tend to follow the same rules as ionic compounds. When naming acids and bases, remember that an acid always contains a hydrogen atom and an ion. To name a base, add the word hydroxide to the name of the.
WritingandNamingAcidsandBasesNotes
An oxyacid is an acid that consists of hydrogen, oxygen, and a. When naming acids and bases, remember that an acid always contains a hydrogen atom and an ion. More practice examples on nomenclature and formulas. To name a base, add the word hydroxide to the name of the cation: Bases tend to follow the same rules as ionic compounds.
Naming Acids Worksheet
The general properties of acids and bases are the following. More practice examples on nomenclature and formulas. When naming acids and bases, remember that an acid always contains a hydrogen atom and an ion. An oxyacid is an acid that consists of hydrogen, oxygen, and a. In simple binary acids, one ion is attached to hydrogen.
Naming Acids and Bases Chemistry Steps
More practice examples on nomenclature and formulas. In simple binary acids, one ion is attached to hydrogen. Bases tend to follow the same rules as ionic compounds. When naming acids and bases, remember that an acid always contains a hydrogen atom and an ion. An oxyacid is an acid that consists of hydrogen, oxygen, and a.
PPT Chapter 14 Acids and Bases PowerPoint Presentation, free download
The general properties of acids and bases are the following. Bases tend to follow the same rules as ionic compounds. More practice examples on nomenclature and formulas. An oxyacid is an acid that consists of hydrogen, oxygen, and a. To name a base, add the word hydroxide to the name of the cation:
Naming Acids and Bases
The general properties of acids and bases are the following. To name a base, add the word hydroxide to the name of the cation: In simple binary acids, one ion is attached to hydrogen. More practice examples on nomenclature and formulas. Bases tend to follow the same rules as ionic compounds.
Common Acids And Bases You Should Know
To name a base, add the word hydroxide to the name of the cation: Bases tend to follow the same rules as ionic compounds. In simple binary acids, one ion is attached to hydrogen. The general properties of acids and bases are the following. An oxyacid is an acid that consists of hydrogen, oxygen, and a.
To Name A Base, Add The Word Hydroxide To The Name Of The Cation:
An oxyacid is an acid that consists of hydrogen, oxygen, and a. Bases tend to follow the same rules as ionic compounds. The general properties of acids and bases are the following. When naming acids and bases, remember that an acid always contains a hydrogen atom and an ion.
More Practice Examples On Nomenclature And Formulas.
In simple binary acids, one ion is attached to hydrogen.