Reaction Rate Worksheet
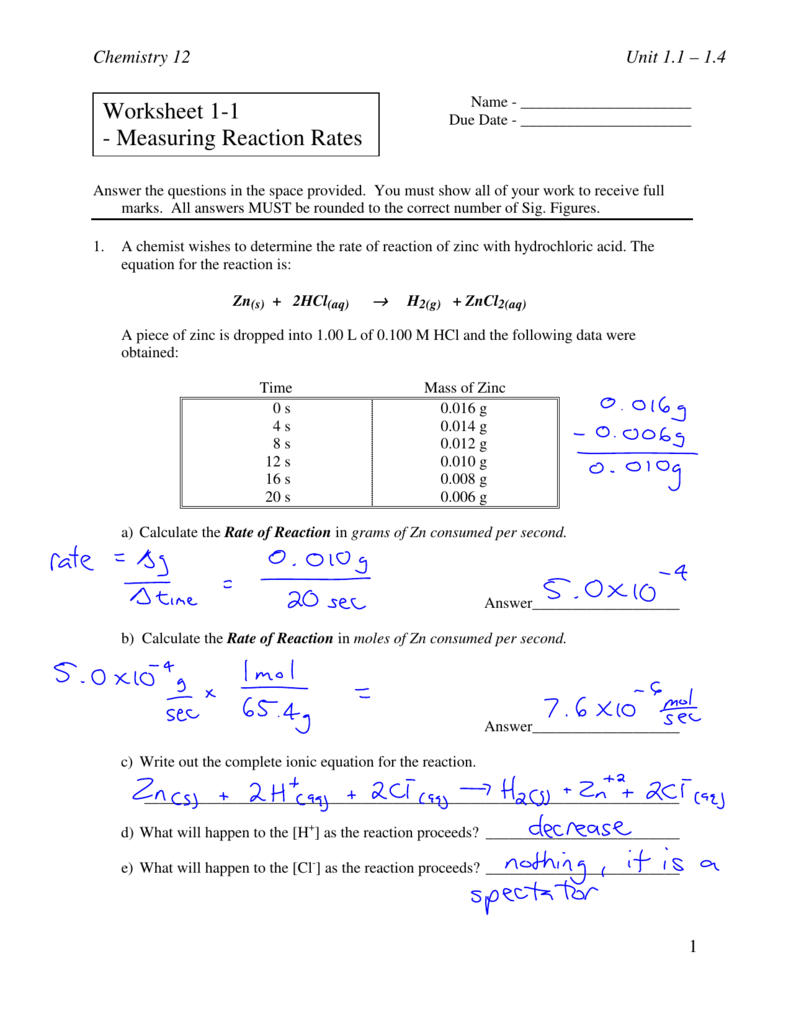
Reaction Rate Worksheet - A chemist wishes to determine the rate of reaction of zinc with hydrochloric acid. Rates of reaction some reactions occur very quickly, like the burning of petrol in air in an engine. Calculate the mean rate of reaction from 0 to 15 seconds. C 6 h 12 o 6 (s) + 6 o 2 (g) 6 h 2 o (g) + 6 co 2 (g) 1. Other reactions can take a very long time, like the. A student investigated the rate of reaction between calcium carbonate (marble chips) and. A study of reaction _____ is called chemical _____. Give some examples of situations where we might want to decrease the rate of a particular reaction. Reaction rate refers to how quickly or slowly the _____ disappear and the _____ appear.
C 6 h 12 o 6 (s) + 6 o 2 (g) 6 h 2 o (g) + 6 co 2 (g) 1. Give some examples of situations where we might want to decrease the rate of a particular reaction. Rates of reaction some reactions occur very quickly, like the burning of petrol in air in an engine. Reaction rate refers to how quickly or slowly the _____ disappear and the _____ appear. A chemist wishes to determine the rate of reaction of zinc with hydrochloric acid. A student investigated the rate of reaction between calcium carbonate (marble chips) and. A study of reaction _____ is called chemical _____. Other reactions can take a very long time, like the. Calculate the mean rate of reaction from 0 to 15 seconds.
Calculate the mean rate of reaction from 0 to 15 seconds. C 6 h 12 o 6 (s) + 6 o 2 (g) 6 h 2 o (g) + 6 co 2 (g) 1. Give some examples of situations where we might want to decrease the rate of a particular reaction. Reaction rate refers to how quickly or slowly the _____ disappear and the _____ appear. Other reactions can take a very long time, like the. A chemist wishes to determine the rate of reaction of zinc with hydrochloric acid. A study of reaction _____ is called chemical _____. A student investigated the rate of reaction between calcium carbonate (marble chips) and. Rates of reaction some reactions occur very quickly, like the burning of petrol in air in an engine.
SOLUTION Factors affecting the rate of reaction worksheet Studypool
A chemist wishes to determine the rate of reaction of zinc with hydrochloric acid. Give some examples of situations where we might want to decrease the rate of a particular reaction. A study of reaction _____ is called chemical _____. A student investigated the rate of reaction between calcium carbonate (marble chips) and. Other reactions can take a very long.
reaction rates
A study of reaction _____ is called chemical _____. Calculate the mean rate of reaction from 0 to 15 seconds. Give some examples of situations where we might want to decrease the rate of a particular reaction. Other reactions can take a very long time, like the. Reaction rate refers to how quickly or slowly the _____ disappear and the.
12 u lesson 6 rates of reaction worksheet Rates of Reaction
A student investigated the rate of reaction between calcium carbonate (marble chips) and. A chemist wishes to determine the rate of reaction of zinc with hydrochloric acid. Give some examples of situations where we might want to decrease the rate of a particular reaction. Rates of reaction some reactions occur very quickly, like the burning of petrol in air in.
SOLUTION Factors affecting the rate of reaction worksheet Studypool
Calculate the mean rate of reaction from 0 to 15 seconds. Other reactions can take a very long time, like the. Give some examples of situations where we might want to decrease the rate of a particular reaction. Rates of reaction some reactions occur very quickly, like the burning of petrol in air in an engine. A chemist wishes to.
Rate of Reaction Grade 8 Worksheet PDF Catalysis Chemical
Give some examples of situations where we might want to decrease the rate of a particular reaction. A chemist wishes to determine the rate of reaction of zinc with hydrochloric acid. Other reactions can take a very long time, like the. Calculate the mean rate of reaction from 0 to 15 seconds. C 6 h 12 o 6 (s) +.
Rate Of Reaction Worksheet Grade 8 Pdf
C 6 h 12 o 6 (s) + 6 o 2 (g) 6 h 2 o (g) + 6 co 2 (g) 1. Calculate the mean rate of reaction from 0 to 15 seconds. A student investigated the rate of reaction between calcium carbonate (marble chips) and. Reaction rate refers to how quickly or slowly the _____ disappear and the.
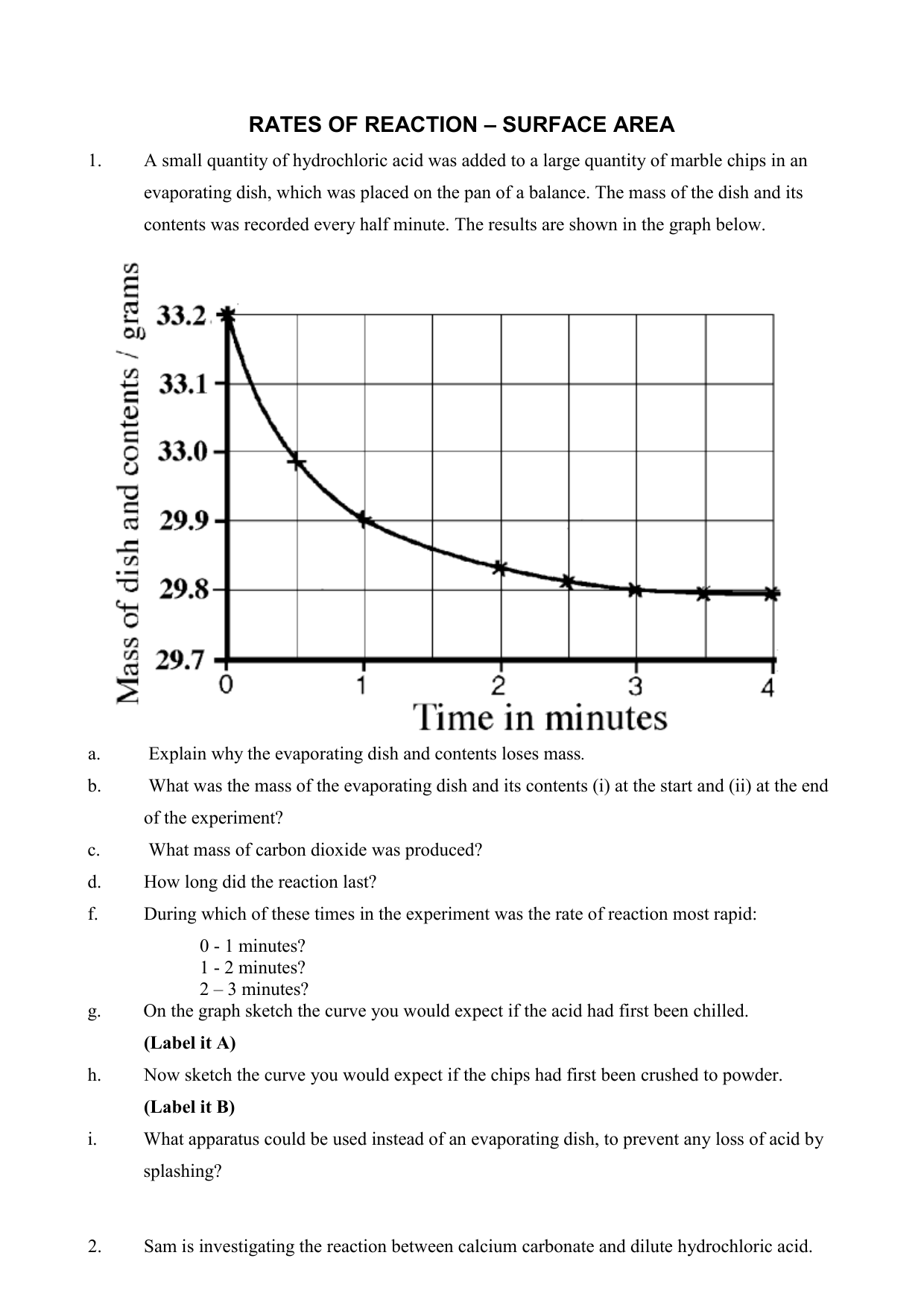
RATES OF REACTION SURFACE AREA
A study of reaction _____ is called chemical _____. Give some examples of situations where we might want to decrease the rate of a particular reaction. Calculate the mean rate of reaction from 0 to 15 seconds. Reaction rate refers to how quickly or slowly the _____ disappear and the _____ appear. Rates of reaction some reactions occur very quickly,.
Rate of Reaction Practical Home Learning Worksheet GCSE rocketsheets
Rates of reaction some reactions occur very quickly, like the burning of petrol in air in an engine. Give some examples of situations where we might want to decrease the rate of a particular reaction. C 6 h 12 o 6 (s) + 6 o 2 (g) 6 h 2 o (g) + 6 co 2 (g) 1. A chemist.
Rate Of Reaction Chemistry Worksheet
A chemist wishes to determine the rate of reaction of zinc with hydrochloric acid. A study of reaction _____ is called chemical _____. Reaction rate refers to how quickly or slowly the _____ disappear and the _____ appear. Calculate the mean rate of reaction from 0 to 15 seconds. Rates of reaction some reactions occur very quickly, like the burning.
Rates Of Reaction Some Reactions Occur Very Quickly, Like The Burning Of Petrol In Air In An Engine.
Calculate the mean rate of reaction from 0 to 15 seconds. A study of reaction _____ is called chemical _____. C 6 h 12 o 6 (s) + 6 o 2 (g) 6 h 2 o (g) + 6 co 2 (g) 1. A student investigated the rate of reaction between calcium carbonate (marble chips) and.
Other Reactions Can Take A Very Long Time, Like The.
Give some examples of situations where we might want to decrease the rate of a particular reaction. Reaction rate refers to how quickly or slowly the _____ disappear and the _____ appear. A chemist wishes to determine the rate of reaction of zinc with hydrochloric acid.