What Occurs When Nacl S Is Added To Water
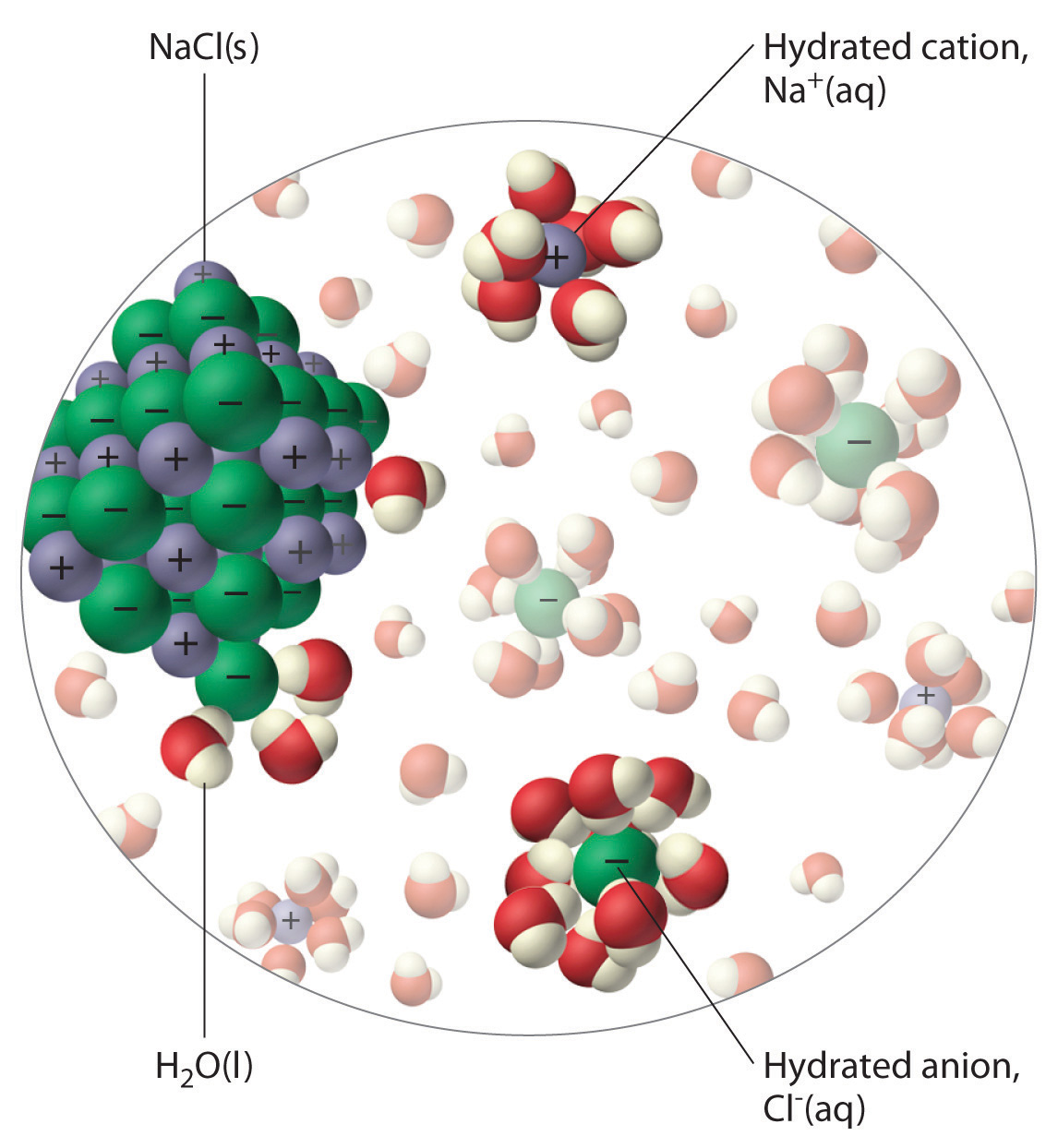
What Occurs When Nacl S Is Added To Water - In chemistry, it results in a solution, as the ionic bond of nacl is pulled. (1) the boiling point of the. Water is attracted to the sodium chloride crystal because water is polar and has both a positive and a negative end. What does salt do in water? When salt (sodium chloride, nacl) is added to water, it dissolves. When table salt, or sodium chloride (nacl), is placed in water, an interesting chemical reaction occurs. Salt is made up of sodium and chloride ions, and. The water molecules surround the sodium chloride crystal. The positively charged sodium ions. When sodium chloride (nacl) dissolves in water, the following steps occur:
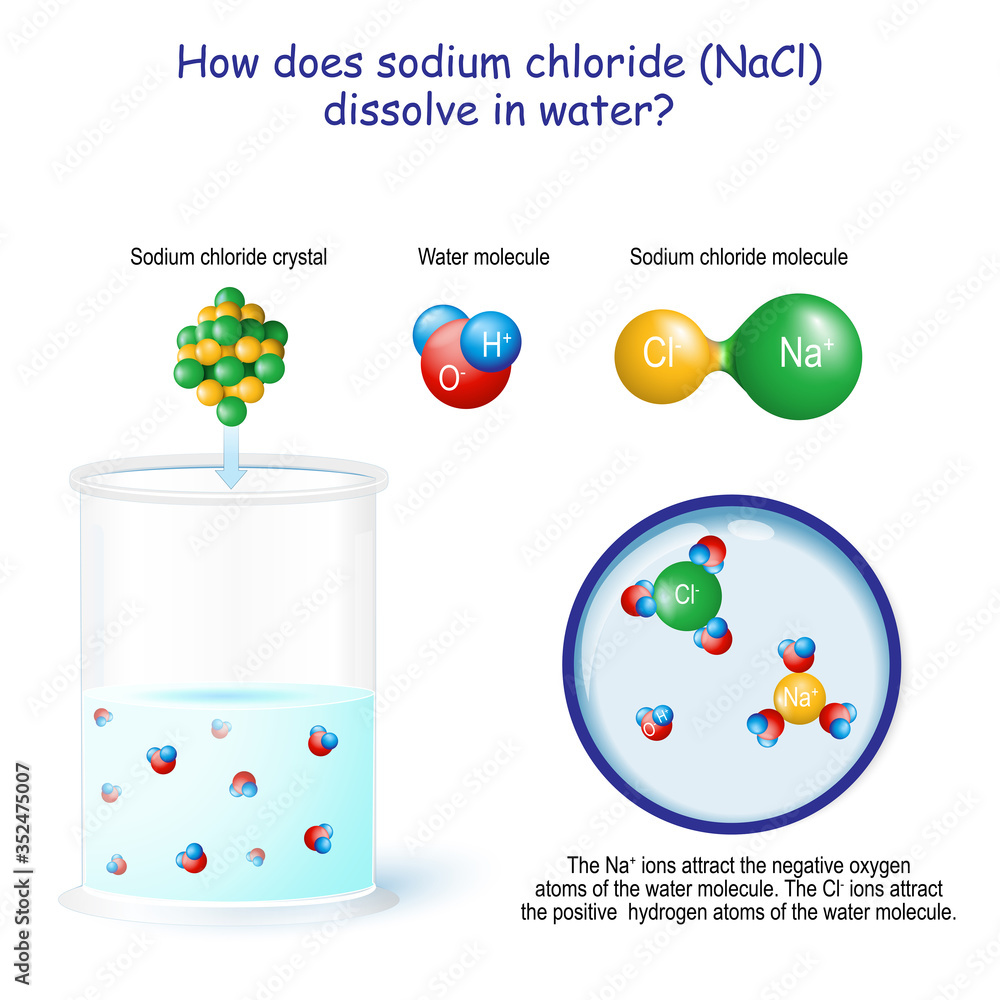
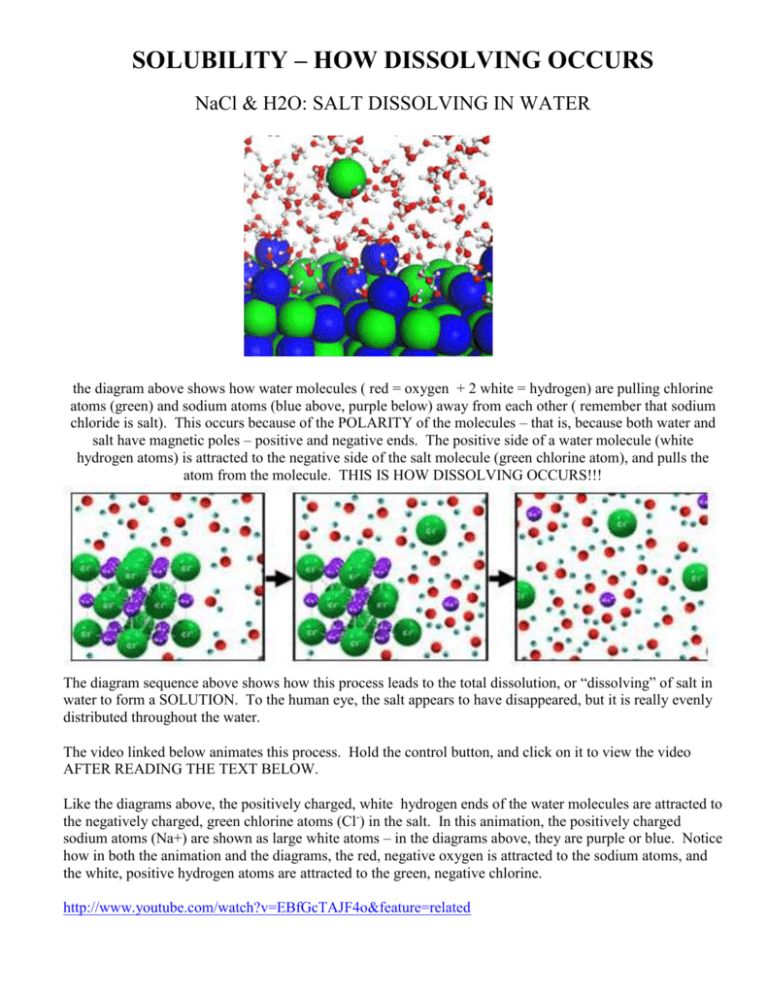
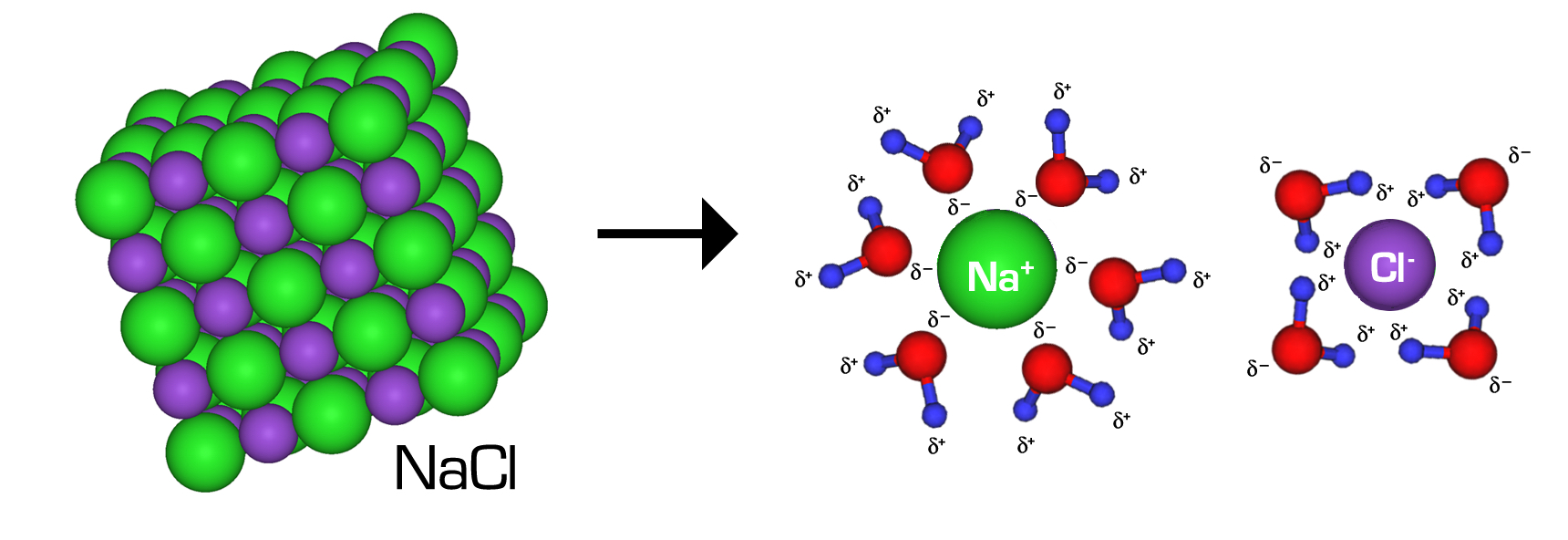
The water molecules surround the sodium chloride crystal. Water is attracted to the sodium chloride crystal because water is polar and has both a positive and a negative end. When table salt, or sodium chloride (nacl), is placed in water, an interesting chemical reaction occurs. Study with quizlet and memorize flashcards containing terms like what occurs when nacl(s) is added to water? The positively charged sodium ions. (1) the boiling point of the. When salt (sodium chloride, nacl) is added to water, it dissolves. In chemistry, it results in a solution, as the ionic bond of nacl is pulled. When sodium chloride (nacl) dissolves in water, the following steps occur: Salt is made up of sodium and chloride ions, and.
(1) the boiling point of the. The water molecules surround the sodium chloride crystal. Water is attracted to the sodium chloride crystal because water is polar and has both a positive and a negative end. In chemistry, it results in a solution, as the ionic bond of nacl is pulled. Salt is made up of sodium and chloride ions, and. What does salt do in water? When salt (sodium chloride, nacl) is added to water, it dissolves. The positively charged sodium ions. When table salt, or sodium chloride (nacl), is placed in water, an interesting chemical reaction occurs. Study with quizlet and memorize flashcards containing terms like what occurs when nacl(s) is added to water?
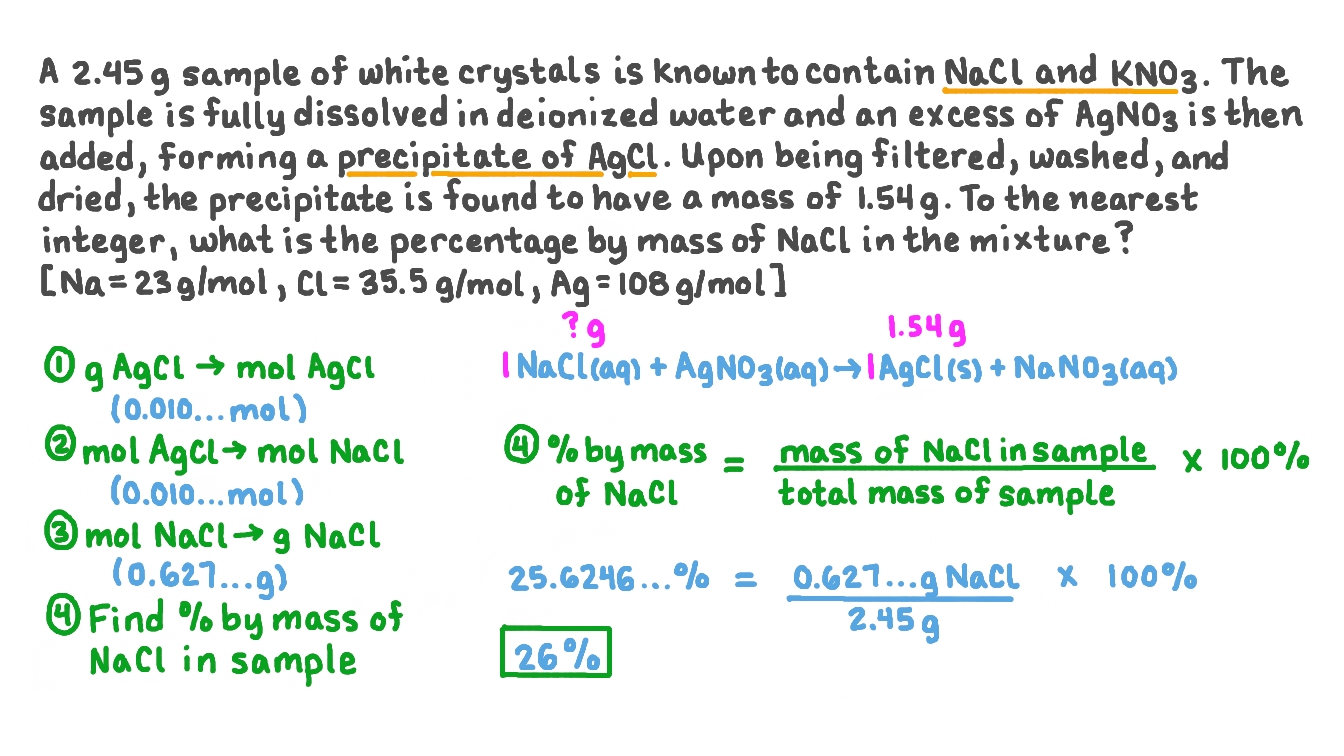
Question Video Using Precipitation Gravimetry to Calculate the
(1) the boiling point of the. Water is attracted to the sodium chloride crystal because water is polar and has both a positive and a negative end. Salt dissolved in water is a rough description of earth's oceans. Study with quizlet and memorize flashcards containing terms like what occurs when nacl(s) is added to water? When salt (sodium chloride, nacl).
How does sodium chloride (NaCl) dissolve in water Векторный объект
In chemistry, it results in a solution, as the ionic bond of nacl is pulled. When table salt, or sodium chloride (nacl), is placed in water, an interesting chemical reaction occurs. Salt dissolved in water is a rough description of earth's oceans. (1) the boiling point of the. When salt (sodium chloride, nacl) is added to water, it dissolves.
SOLVED*4.84. Complete and balance the molecular equations for the
When table salt, or sodium chloride (nacl), is placed in water, an interesting chemical reaction occurs. When salt (sodium chloride, nacl) is added to water, it dissolves. Study with quizlet and memorize flashcards containing terms like what occurs when nacl(s) is added to water? Water is attracted to the sodium chloride crystal because water is polar and has both a.
solubility how dissolving occurs
When sodium chloride (nacl) dissolves in water, the following steps occur: Salt is made up of sodium and chloride ions, and. (1) the boiling point of the. The positively charged sodium ions. When salt (sodium chloride, nacl) is added to water, it dissolves.
Why Does Nacl Dissolve In Water Water Ionizer
The water molecules surround the sodium chloride crystal. The positively charged sodium ions. Study with quizlet and memorize flashcards containing terms like what occurs when nacl(s) is added to water? (1) the boiling point of the. Water is attracted to the sodium chloride crystal because water is polar and has both a positive and a negative end.
SOLVED Calculate the Osmolarity and Osmolality of NaCl in 0.9 (w/v
In chemistry, it results in a solution, as the ionic bond of nacl is pulled. When sodium chloride (nacl) dissolves in water, the following steps occur: Water is attracted to the sodium chloride crystal because water is polar and has both a positive and a negative end. Salt is made up of sodium and chloride ions, and. When salt (sodium.
Question Video Writing a Net Ionic Equation for the Reaction of
Salt is made up of sodium and chloride ions, and. The water molecules surround the sodium chloride crystal. Water is attracted to the sodium chloride crystal because water is polar and has both a positive and a negative end. When sodium chloride (nacl) dissolves in water, the following steps occur: The positively charged sodium ions.
Aqueous Solutions
When table salt, or sodium chloride (nacl), is placed in water, an interesting chemical reaction occurs. When sodium chloride (nacl) dissolves in water, the following steps occur: (1) the boiling point of the. The water molecules surround the sodium chloride crystal. Study with quizlet and memorize flashcards containing terms like what occurs when nacl(s) is added to water?
Nacl Is Not an Organic Molecule
The positively charged sodium ions. Study with quizlet and memorize flashcards containing terms like what occurs when nacl(s) is added to water? When salt (sodium chloride, nacl) is added to water, it dissolves. (1) the boiling point of the. The water molecules surround the sodium chloride crystal.
Solved Write the net ionic equation for the reaction between
When sodium chloride (nacl) dissolves in water, the following steps occur: Salt is made up of sodium and chloride ions, and. The water molecules surround the sodium chloride crystal. When table salt, or sodium chloride (nacl), is placed in water, an interesting chemical reaction occurs. Study with quizlet and memorize flashcards containing terms like what occurs when nacl(s) is added.
In Chemistry, It Results In A Solution, As The Ionic Bond Of Nacl Is Pulled.
When sodium chloride (nacl) dissolves in water, the following steps occur: Salt is made up of sodium and chloride ions, and. The water molecules surround the sodium chloride crystal. When salt (sodium chloride, nacl) is added to water, it dissolves.
(1) The Boiling Point Of The.
When table salt, or sodium chloride (nacl), is placed in water, an interesting chemical reaction occurs. Salt dissolved in water is a rough description of earth's oceans. What does salt do in water? Study with quizlet and memorize flashcards containing terms like what occurs when nacl(s) is added to water?
The Positively Charged Sodium Ions.
Water is attracted to the sodium chloride crystal because water is polar and has both a positive and a negative end.