What Was Wrong With Rutherford S Model Of The Atom
What Was Wrong With Rutherford S Model Of The Atom - The rutherford atomic model was correct in that the atom is mostly empty space. One of the significant flaws in rutherford’s model is its inability to. Rutherford's model of the atom, also known as the nuclear model, was a significant advancement in our understanding of the atomic structure. Most of the mass is in the nucleus, and the nucleus is positively. Cons of rutherford’s atomic model. Inability to explain electron stability. The following are some reasons why rutherford's model was proven wrong:
Rutherford's model of the atom, also known as the nuclear model, was a significant advancement in our understanding of the atomic structure. One of the significant flaws in rutherford’s model is its inability to. The following are some reasons why rutherford's model was proven wrong: Most of the mass is in the nucleus, and the nucleus is positively. Cons of rutherford’s atomic model. The rutherford atomic model was correct in that the atom is mostly empty space. Inability to explain electron stability.
Rutherford's model of the atom, also known as the nuclear model, was a significant advancement in our understanding of the atomic structure. Inability to explain electron stability. Most of the mass is in the nucleus, and the nucleus is positively. One of the significant flaws in rutherford’s model is its inability to. The rutherford atomic model was correct in that the atom is mostly empty space. Cons of rutherford’s atomic model. The following are some reasons why rutherford's model was proven wrong:
What Is Rutherford’s atomic model? What are the reasons for failure?
Most of the mass is in the nucleus, and the nucleus is positively. Cons of rutherford’s atomic model. The following are some reasons why rutherford's model was proven wrong: The rutherford atomic model was correct in that the atom is mostly empty space. One of the significant flaws in rutherford’s model is its inability to.
What Was Wrong With Rutherford's Model of the Atom
Most of the mass is in the nucleus, and the nucleus is positively. Rutherford's model of the atom, also known as the nuclear model, was a significant advancement in our understanding of the atomic structure. Inability to explain electron stability. Cons of rutherford’s atomic model. One of the significant flaws in rutherford’s model is its inability to.
Which Best Describes Rutherford's Model of the Atom
Most of the mass is in the nucleus, and the nucleus is positively. Cons of rutherford’s atomic model. The following are some reasons why rutherford's model was proven wrong: Rutherford's model of the atom, also known as the nuclear model, was a significant advancement in our understanding of the atomic structure. The rutherford atomic model was correct in that the.
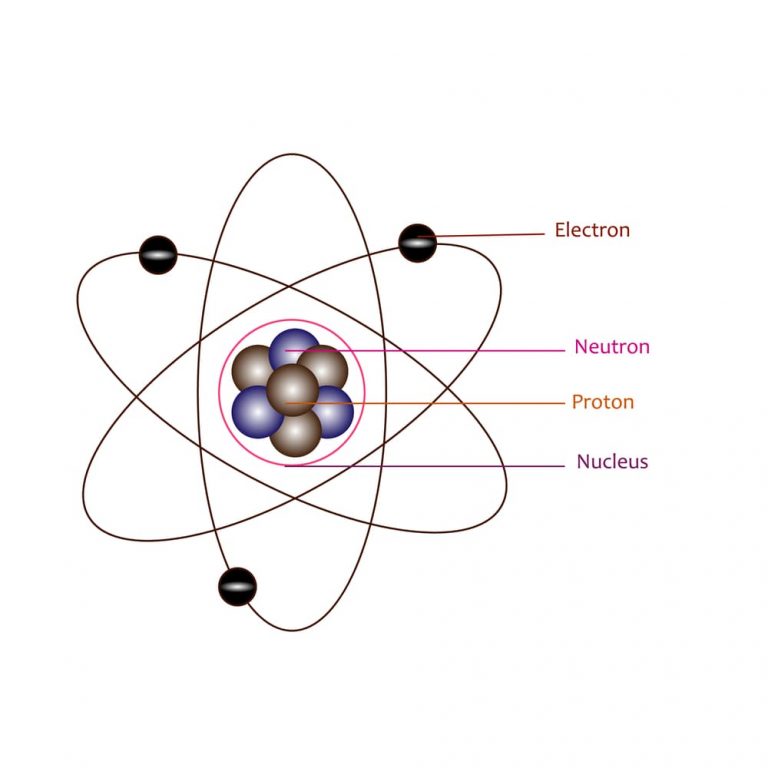
Rutherford’s Atomic Model Part 1 Atoms and Molecules Infinity
One of the significant flaws in rutherford’s model is its inability to. Rutherford's model of the atom, also known as the nuclear model, was a significant advancement in our understanding of the atomic structure. Cons of rutherford’s atomic model. The rutherford atomic model was correct in that the atom is mostly empty space. Most of the mass is in the.
Who drew the first model of the atom?
Rutherford's model of the atom, also known as the nuclear model, was a significant advancement in our understanding of the atomic structure. The following are some reasons why rutherford's model was proven wrong: Most of the mass is in the nucleus, and the nucleus is positively. The rutherford atomic model was correct in that the atom is mostly empty space..
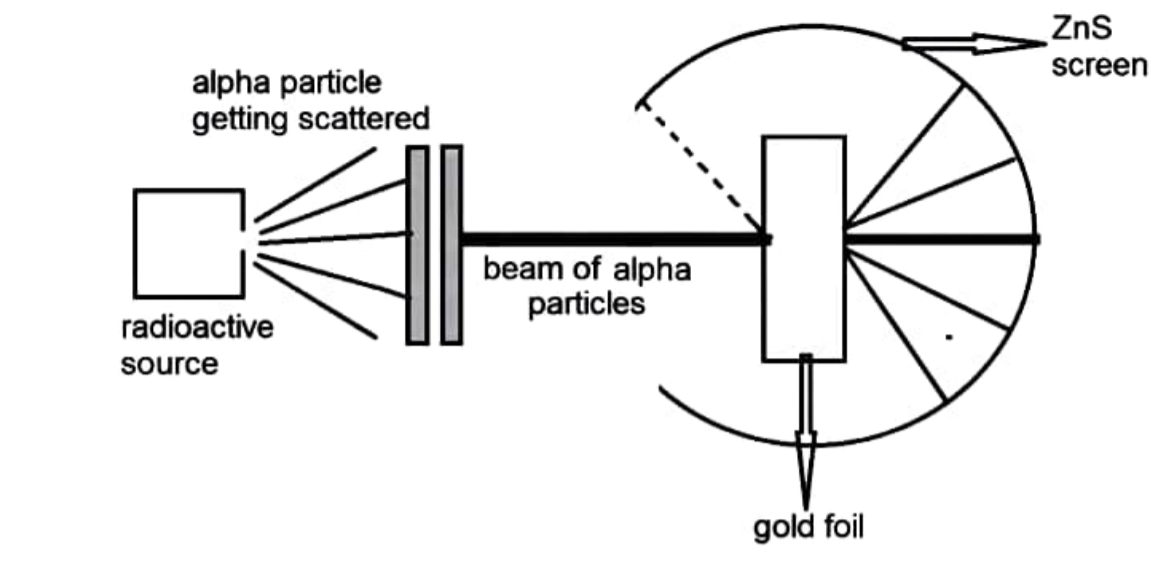
Rutherford Model of Atom Scattering Experiment Structure of Atom
Cons of rutherford’s atomic model. One of the significant flaws in rutherford’s model is its inability to. Inability to explain electron stability. The following are some reasons why rutherford's model was proven wrong: The rutherford atomic model was correct in that the atom is mostly empty space.
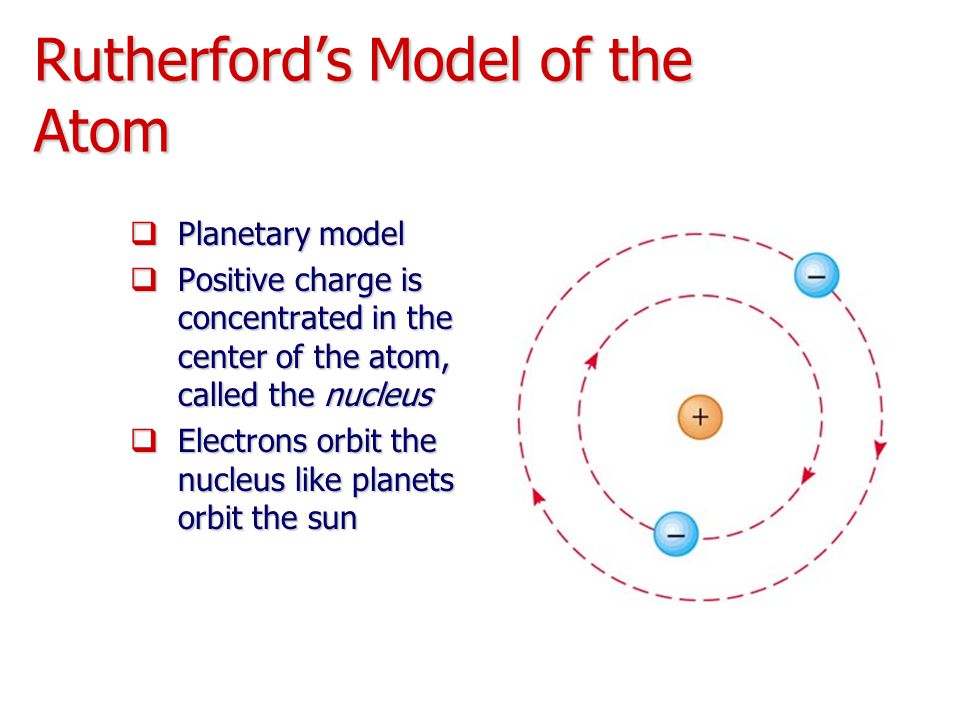
Rutherford’s atomic model the nuclear atom Pharmacy Gyan
Inability to explain electron stability. One of the significant flaws in rutherford’s model is its inability to. Most of the mass is in the nucleus, and the nucleus is positively. Rutherford's model of the atom, also known as the nuclear model, was a significant advancement in our understanding of the atomic structure. The rutherford atomic model was correct in that.
ALI BABA Rutherford's Model of the Atom
The following are some reasons why rutherford's model was proven wrong: Most of the mass is in the nucleus, and the nucleus is positively. Cons of rutherford’s atomic model. Rutherford's model of the atom, also known as the nuclear model, was a significant advancement in our understanding of the atomic structure. Inability to explain electron stability.
Rutherford model Definition & Facts Britannica
One of the significant flaws in rutherford’s model is its inability to. Cons of rutherford’s atomic model. Inability to explain electron stability. Rutherford's model of the atom, also known as the nuclear model, was a significant advancement in our understanding of the atomic structure. The following are some reasons why rutherford's model was proven wrong:
Pengertian Atom dan Perkembangan Atom Berdasarkan Teorinya Gramedia
The following are some reasons why rutherford's model was proven wrong: Most of the mass is in the nucleus, and the nucleus is positively. The rutherford atomic model was correct in that the atom is mostly empty space. Rutherford's model of the atom, also known as the nuclear model, was a significant advancement in our understanding of the atomic structure..
The Following Are Some Reasons Why Rutherford's Model Was Proven Wrong:
Inability to explain electron stability. One of the significant flaws in rutherford’s model is its inability to. Most of the mass is in the nucleus, and the nucleus is positively. Rutherford's model of the atom, also known as the nuclear model, was a significant advancement in our understanding of the atomic structure.
Cons Of Rutherford’s Atomic Model.
The rutherford atomic model was correct in that the atom is mostly empty space.