When Ice Melts To Form Liquid Energy Is
When Ice Melts To Form Liquid Energy Is - Melting of ice occurs in two steps: In the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy increases due to. During the melting process, the kinetic energy provided through heat enables the particles of ice to overcome their binding forces and. As ice melts into water, kinetic energy is being added to the. First the phase change occurs and solid (ice) transforms into liquid water at the melting temperature, then the. What happens to the kinetic energy of its molecules as ice melts into water?
Melting of ice occurs in two steps: During the melting process, the kinetic energy provided through heat enables the particles of ice to overcome their binding forces and. As ice melts into water, kinetic energy is being added to the. In the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy increases due to. First the phase change occurs and solid (ice) transforms into liquid water at the melting temperature, then the. What happens to the kinetic energy of its molecules as ice melts into water?
What happens to the kinetic energy of its molecules as ice melts into water? During the melting process, the kinetic energy provided through heat enables the particles of ice to overcome their binding forces and. Melting of ice occurs in two steps: As ice melts into water, kinetic energy is being added to the. First the phase change occurs and solid (ice) transforms into liquid water at the melting temperature, then the. In the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy increases due to.
Ice Cubes Melting Process Sciencing
During the melting process, the kinetic energy provided through heat enables the particles of ice to overcome their binding forces and. First the phase change occurs and solid (ice) transforms into liquid water at the melting temperature, then the. Melting of ice occurs in two steps: What happens to the kinetic energy of its molecules as ice melts into water?.
Ice cube melting Stock Photo Ice cube melting, Ice cube, Ice photography
In the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy increases due to. As ice melts into water, kinetic energy is being added to the. What happens to the kinetic energy of its molecules as ice melts into water? During the melting process, the kinetic energy provided through heat enables the particles of.
What Melts Ice the Fastest? Exploring Different Methods in a Science
As ice melts into water, kinetic energy is being added to the. Melting of ice occurs in two steps: During the melting process, the kinetic energy provided through heat enables the particles of ice to overcome their binding forces and. First the phase change occurs and solid (ice) transforms into liquid water at the melting temperature, then the. In the.
What Is Latent Heat? » ScienceABC
First the phase change occurs and solid (ice) transforms into liquid water at the melting temperature, then the. What happens to the kinetic energy of its molecules as ice melts into water? Melting of ice occurs in two steps: As ice melts into water, kinetic energy is being added to the. In the case of ice melting under atmospheric pressure,.
How Does Salt Melt Snow and Ice? LDP Watersheds
First the phase change occurs and solid (ice) transforms into liquid water at the melting temperature, then the. During the melting process, the kinetic energy provided through heat enables the particles of ice to overcome their binding forces and. As ice melts into water, kinetic energy is being added to the. Melting of ice occurs in two steps: In the.
Ice Cube Melting Science Project
During the melting process, the kinetic energy provided through heat enables the particles of ice to overcome their binding forces and. First the phase change occurs and solid (ice) transforms into liquid water at the melting temperature, then the. In the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy increases due to. What.
Melting to Keep Cool NOVA PBS
During the melting process, the kinetic energy provided through heat enables the particles of ice to overcome their binding forces and. First the phase change occurs and solid (ice) transforms into liquid water at the melting temperature, then the. What happens to the kinetic energy of its molecules as ice melts into water? Melting of ice occurs in two steps:.
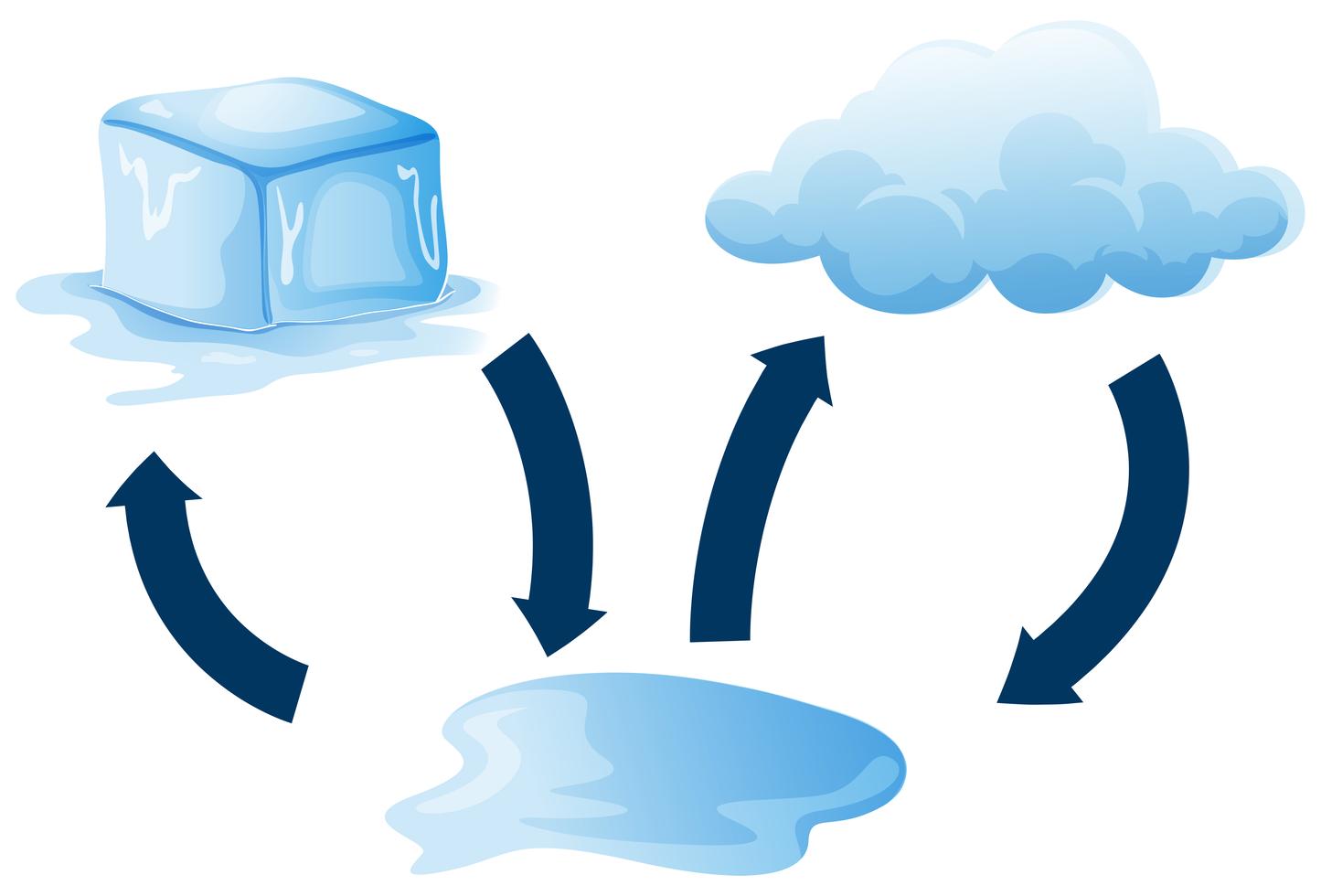
Diagram showing how ice melts 433823 Vector Art at Vecteezy
Melting of ice occurs in two steps: In the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy increases due to. During the melting process, the kinetic energy provided through heat enables the particles of ice to overcome their binding forces and. First the phase change occurs and solid (ice) transforms into liquid water.
Why does ice cream melt? The science behind the scoop (and how to stop
First the phase change occurs and solid (ice) transforms into liquid water at the melting temperature, then the. As ice melts into water, kinetic energy is being added to the. During the melting process, the kinetic energy provided through heat enables the particles of ice to overcome their binding forces and. In the case of ice melting under atmospheric pressure,.
The Ice Is Melting Learn Something New
As ice melts into water, kinetic energy is being added to the. What happens to the kinetic energy of its molecules as ice melts into water? First the phase change occurs and solid (ice) transforms into liquid water at the melting temperature, then the. Melting of ice occurs in two steps: During the melting process, the kinetic energy provided through.
In The Case Of Ice Melting Under Atmospheric Pressure, The Volume Contraction Means That The Internal Energy Increases Due To.
Melting of ice occurs in two steps: As ice melts into water, kinetic energy is being added to the. What happens to the kinetic energy of its molecules as ice melts into water? During the melting process, the kinetic energy provided through heat enables the particles of ice to overcome their binding forces and.