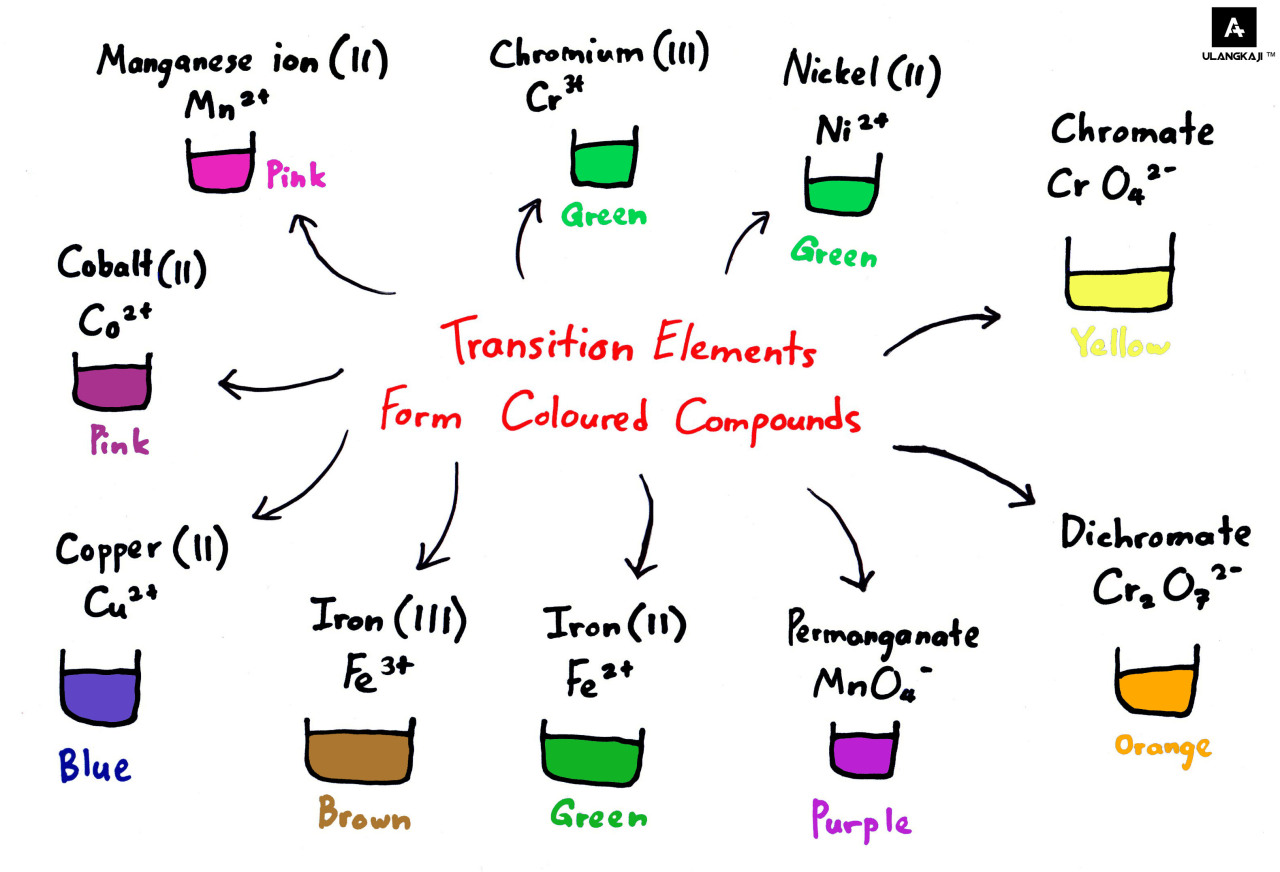
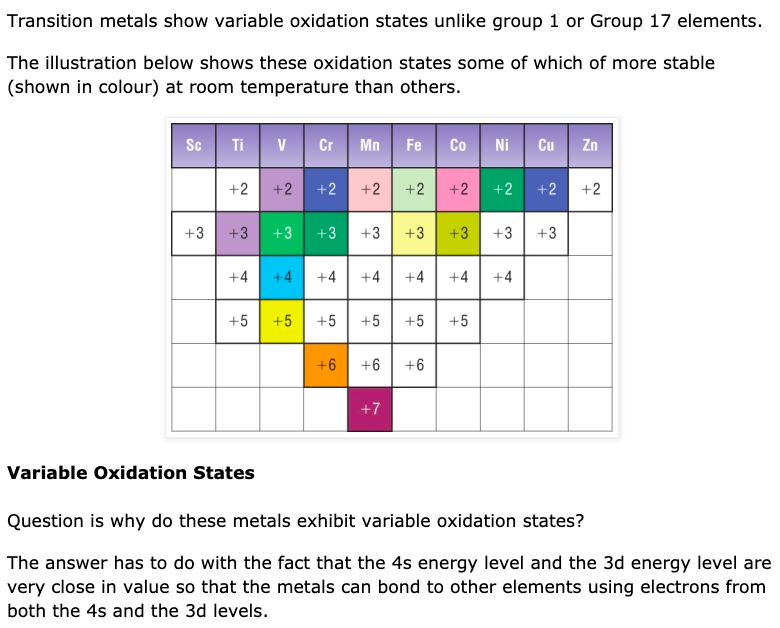
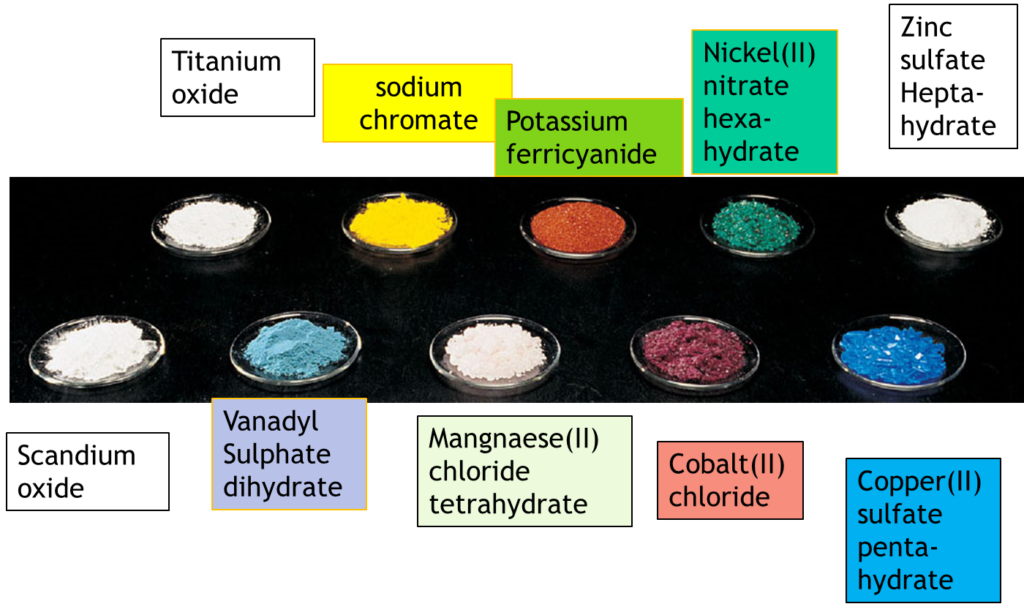
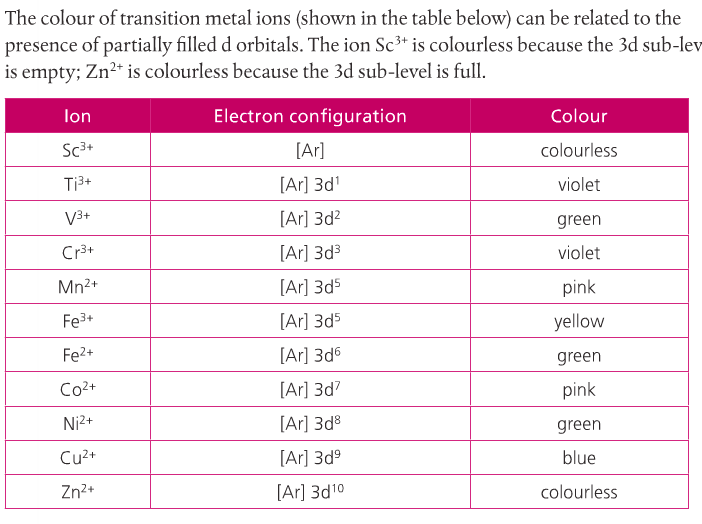
Why Transition Metals Form Coloured Compounds
Why Transition Metals Form Coloured Compounds - In their lower oxidation states, the transition elements form ionic compounds; In their higher oxidation states, they form covalent.
In their lower oxidation states, the transition elements form ionic compounds; In their higher oxidation states, they form covalent.
In their higher oxidation states, they form covalent. In their lower oxidation states, the transition elements form ionic compounds;
Explain how transition elements form coloured compounds?
In their higher oxidation states, they form covalent. In their lower oxidation states, the transition elements form ionic compounds;
PPT Atoms and ElementsThe Nature of Matter PowerPoint Presentation
In their higher oxidation states, they form covalent. In their lower oxidation states, the transition elements form ionic compounds;
The transition metals generally form coloured compounds. Transition meta..
In their higher oxidation states, they form covalent. In their lower oxidation states, the transition elements form ionic compounds;
Compound Interest Colours of Transition Metal Ions in Aqueous Solution
In their higher oxidation states, they form covalent. In their lower oxidation states, the transition elements form ionic compounds;
Transition Metals as Colored Compounds
In their lower oxidation states, the transition elements form ionic compounds; In their higher oxidation states, they form covalent.
Why do Transition Metals Form Coloured Compounds? YouTube
In their lower oxidation states, the transition elements form ionic compounds; In their higher oxidation states, they form covalent.
SPMStraightA — Transition Metals make coloured compounds
In their lower oxidation states, the transition elements form ionic compounds; In their higher oxidation states, they form covalent.
13 Transition Metals & Colored Complexes The!Mad!Scientist!
In their lower oxidation states, the transition elements form ionic compounds; In their higher oxidation states, they form covalent.
Transition metals W3schools
In their lower oxidation states, the transition elements form ionic compounds; In their higher oxidation states, they form covalent.
In Their Higher Oxidation States, They Form Covalent.
In their lower oxidation states, the transition elements form ionic compounds;